Short-course oral steroids alone for chronic rhinosinusitis
- PMID: 27113367
- PMCID: PMC8504433
- DOI: 10.1002/14651858.CD011991.pub2
Short-course oral steroids alone for chronic rhinosinusitis
Abstract
Background: This review is one of a suite of six Cochrane reviews looking at the primary medical management options for patients with chronic rhinosinusitis.Chronic rhinosinusitis is a common condition involving inflammation of the lining of the nose and paranasal sinuses. It is characterised by nasal blockage and nasal discharge, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. Oral corticosteroids are used to control the inflammatory response and improve symptoms.
Objectives: To assess the effects of oral corticosteroids compared with placebo/no intervention or other pharmacological interventions (intranasal corticosteroids, antibiotics, antifungals) for chronic rhinosinusitis.
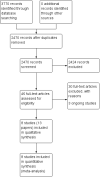
Search methods: The Cochrane ENT Information Specialist searched the ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2015, Issue 7); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 11 August 2015.
Selection criteria: Randomised controlled trials (RCTs) comparing a short course (up to 21 days) of oral corticosteroids with placebo or no treatment or compared with other pharmacological interventions.
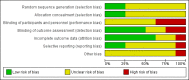
Data collection and analysis: We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease-specific health-related quality of life (HRQL), patient-reported disease severity, and the adverse event of mood or behavioural disturbances. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse events of insomnia, gastrointestinal disturbances and osteoporosis. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
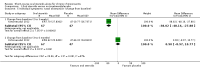
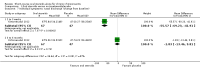
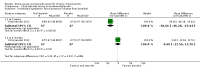
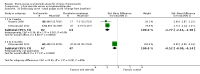
Main results: We included eight RCTs (474 randomised participants), which compared oral corticosteroids with placebo or no intervention. All trials only recruited adults with chronic rhinosinusitis with nasal polyps. All trials reported outcomes at two to three weeks, at the end of the short-course oral steroid treatment period. Three trials additionally reported outcomes at three to six months. Two of these studies prescribed intranasal steroids to patients in both arms of the trial at the end of the oral steroid treatment period. Oral steroids versus placebo or no intervention Disease-specific health-related quality of life was reported by one study. This study reported improved quality of life after treatment (two to three weeks) in the group receiving oral steroids compared with the group who received placebo (standardised mean difference (SMD) -1.24, 95% confidence interval (CI) -1.92 to -0.56, 40 participants, modified RSOM-31), which corresponds to a large effect size. We assessed the evidence to be low quality (we are uncertain about the effect estimate; the true effect may be substantially different from the estimate of the effect). Disease severity as measured by patient-reported symptom scores was reported by two studies, which allowed the four key symptoms used to define chronic rhinosinusitis (nasal blockage, nasal discharge, facial pressure, hyposmia) to be combined into one score. The results at the end of treatment (two to three weeks) showed an improvement in patients receiving oral steroids compared to placebo, both when presented as a mean final value (SMD -2.84, 95% CI -4.09 to -1.59, 22 participants) and as a change from baseline (SMD -2.28, 95% CI -2.76 to -1.80, 114 participants). These correspond to large effect sizes but we assessed the evidence to be low quality.One study (114 participants) followed patients for 10 weeks after the two-week treatment period. All patients in both arms received intranasal steroids at the end of the oral steroid treatment period. The results showed that the initial results after treatment were not sustained (SMD -0.22, 95% CI -0.59 to 0.15, 114 participants, percentage improvement from baseline). This corresponds to a small effect size and we assessed the evidence to be low quality.There was an increase in adverse events in people receiving orals steroids compared with placebo for gastrointestinal disturbances (risk ratio (RR) 3.45, 95% CI 1.11 to 10.78; 187 participants; three studies) and insomnia (RR 3.63, 95% CI 1.10 to 11.95; 187 participants; three studies). There was no significant impact of oral steroids on mood disturbances at the dosage used in the included study (risk ratio (RR) 2.50, 95% CI 0.55 to 11.41; 40 participants; one study). We assessed the evidence to be low quality due to the lack of definitions of the adverse events and the small number of events or sample size, or both). Other comparisons No studies that compared short-course oral steroids with other treatment for chronic rhinosinusitis met the inclusion criteria.
Authors' conclusions: At the end of the treatment course (two to three weeks) there is an improvement in health-related quality of life and symptom severity in patients with chronic rhinosinusitis with nasal polyps taking oral corticosteroids compared with placebo or no treatment. The quality of the evidence supporting this finding is low. At three to six months after the end of the oral steroid treatment period, there is little or no improvement in health-related quality of life or symptom severity for patients taking an initial course of oral steroids compared with placebo or no treatment.The data on the adverse effects associated with short courses of oral corticosteroids indicate that there may be an increase in insomnia and gastrointestinal disturbances but it is not clear whether there is an increase in mood disturbances. All of the adverse events results are based on low quality evidence.More research in this area, particularly research evaluating patients with chronic rhinosinusitis without nasal polyps, longer-term outcomes and adverse effects, is required.There is no evidence for oral steroids compared with other treatments.
Conflict of interest statement
Lee Yee Chong: none known.
Karen Head: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
Figures















Update of
References
References to studies included in this review
Alobid 2014 {published data only}
-
- Alobid I, Benitez P, Valero A, Munoz R, Langdon C. Oral and intranasal steroid treatments improve nasal patency and paradoxically increase nasal nitric oxide in patients with severe nasal polyposis. Rhinology 2012;2:171‐7. - PubMed
-
- Alobid I, Benítez P, Cardelús S, Borja Callejas F, Lehrer‐Coriat E, Pujols L, et al. Oral plus nasal corticosteroids improve smell, nasal congestion, and inflammation in sino‐nasal polyposis. Laryngoscope 2014;124(1):50‐6. - PubMed
Benitez 2006 {published data only}
-
- Alobid I, Benitez P, Pujols L, Maldonado M, Bernal‐Sprekelsen M, Morello A, et al. Severe nasal polyposis and its impact on quality of life. The effect of a short course of oral steroids followed by long‐term intranasal steroid treatment. Rhinology 2006;44(1):8‐13. - PubMed
-
- Benitez P, Alobid I, Haro J, Berenguer J, Bernal‐Sprekelsen, Pujols L, et al. A short course of oral prednisone followed by intranasal budesonide is an effective treatment of severe nasal polyps. Laryngoscope 2006;116(5):770‐5. - PubMed
-
- Martínez‐Antón A, Bolós C, Alobid I, Benítez P, Roca‐Ferrer J, Picado C, et al. Corticosteroid therapy increases membrane‐tethered while decreases secreted mucin expression in nasal polyps. Allergy: European Journal of Allergy and Clinical Immunology 2008;63(10):1368‐76. - PubMed
Ecevit 2015 {published and unpublished data}
Hissaria 2006 {published data only}
-
- Hissaria P, Smith W, Wormald PJ, Taylor J, Vadas M, Gillis D, et al. Short course of systemic corticosteroids in sinonasal polyposis: a double‐blind, randomized, placebo‐controlled trial with evaluation of outcome measures. Journal of Allergy and Clinical Immunology 2006;118(1):128‐33. - PubMed
Kapucu 2012 {published data only}
-
- Kapucu B, Cekin E, Erkul BE, Cincik H, Gungor A, Berber U. The effects of systemic, topical, and intralesional steroid treatments on apoptosis level of nasal polyps. Otolaryngology ‐ Head and Neck Surgery 2012;147(3):563‐7. - PubMed
Kirtsreesakul 2012 {published data only}
-
- Kirtsreesakul V, Wongsritrang K, Ruttanaphol S. Clinical efficacy of a short course of systemic steroids in nasal polyposis. Rhinology 2011;49(5):525‐32. - PubMed
Vaidyanathan 2011 {published data only}
-
- Vaidyanathan S, Barnes M, Williamson P, Hopkinson P, Donnan PT, Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Annals of Internal Medicine 2011;154:293‐302. - PubMed
Van Zele 2010 {published data only (unpublished sought but not used)}
-
- Zele T, Gevaert P, Holtappels G. Treatment of nasal polyposis with oral methylprednisolone: a double‐blind, randomized, placebo‐controlled trial with evaluation of clinical and biological activity. Journal of Allergy and Clinical Immunology 2008;121(2 Suppl 1):S265.
-
- Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. Journal of Allergy and Clinical Immunology 2010;125(5):1069‐76.e4. - PubMed
References to studies excluded from this review
Alobid 2005 {published data only}
-
- Alobid I, Benítez P, Bernal‐Sprekelsen M, Roca J, Alonso J, Picado C, et al. Nasal polyposis and its impact on quality of life: comparison between the effects of medical and surgical treatments. Allergy 2005;60(4):452‐8. - PubMed
Blomqvist 2001 {published data only}
-
- Blomqvist EH, Lundblad L, Anggard A, Haraldsson P‐O, Stjarne P. A randomized controlled study evaluating medical treatment versus surgical treatment in addition to medical treatment of nasal polyposis. Journal of Allergy and Clinical Immunology 2001;107(2):224‐8. - PubMed
Blomqvist 2009 {published data only}
-
- Blomqvist EH, Lundblad L, Bergstedt H, Stjarne P. A randomized prospective study comparing medical and medical‐surgical treatment of nasal polyposis by CT. Acta Oto‐Laryngologica 2009;129(5):545‐9. - PubMed
Bonfils 1998 {published data only}
-
- Bonfils P. Medical treatment of paranasal sinus polyposis: a prospective study in 181 patients [Le traitement medical de la polypose naso‐sinusienne: etude prospective sur une serie de 181 patients]. Annales d'Oto‐Laryngologie et de Chirurgie Cervico Faciale 1998;115(4):202‐14. - PubMed
Bonfils 2003 {published data only}
-
- Bonfils P, Nores J‐M, Halimi P, Avan P. Medical treatment of stage I nasal polyposis over a 3‐year follow‐up period. ORL; Journal of Oto‐Rhino‐Laryngology and Its Related Specialties 2004;66(1):27‐34. - PubMed
Bonfils 2006 {published data only}
-
- Bonfils P, Halimi P, Malinvaud D. Adrenal suppression and osteoporosis after treatment of nasal polyposis. Acta Oto‐Laryngologica 2006;126(11):1195‐200. - PubMed
Bülbül 2013 {published data only}
-
- Bülbül T, Bülbül OG, Güçlü O, Bilsel AS, Gürsan SÖ. Effect of glucocorticoids on nasal polyposis, with detection of inflammatory response by measurement of nitric oxide levels in nasal polyp tissue. Journal of Laryngology and Otology 2013;127(6):584‐9. - PubMed
Chi Chan 1996 {published data only}
-
- Chi Chan A, Couto y Arcos F, Martin Biasotti F, Bross Soriano D, Vazquez Valle MDC, Gonzalez Olvera S. Oral steroids as preoperative medication in nasal polyposis [Esteroides orales en la preparacion preoperatoria de poliposis nasal]. Anales de Otorrinolaringologia Mexicana 1996;41(3):155‐60.
Damm 1999 {published data only}
-
- Damm M, Jungehulsing M, Eckel HE, Schmidt M, Theissen P. Effects of systemic steroid treatment in chronic polypoid rhinosinusitis evaluated with magnetic resonance imaging. Otolaryngology ‐ Head and Neck Surgery 1999;120(4):517‐23. - PubMed
Grammer 2013 {published data only}
-
- Grammer LC. Doxycycline or oral corticosteroids for nasal polyps. Journal of Allergy and Clinical Immunology. In Practice 2013;1(5):541‐2. - PubMed
Hessler 2007 {published data only}
-
- Hessler JL, Piccirillo JF, Fang D, Vlahiotis A, Banerji A, Levitt RG, et al. Clinical outcomes of chronic rhinosinusitis in response to medical therapy: results of a prospective study. American Journal of Rhinology 2007;21(1):10‐8. - PubMed
Jankowski 2003a {published data only}
-
- Jankowski R, Bodino C. Evolution of symptoms associated to nasal polyposis following oral steroid treatment and nasalisation of the ethmoid ‐ radical ethmoidectomy is functional surgery for NPS. Rhinology 2003;41(4):211‐9. - PubMed
Jankowski 2003b {published data only}
-
- Jankowski R, Bodino C. Olfaction in patients with nasal polyposis: effects of systemic steroids and radical ethmoidectomy with middle turbinate resection (nasalisation). Rhinology 2003;41(4):220‐30. - PubMed
Kroflic 2006 {published data only}
-
- Kroflic B, Baudoin T, Kalogjera L. Topical furosemide versus oral steroid in preoperative management of nasal polyposis. European Archives of Oto‐Rhino‐Laryngology 2006;263(8):767‐71. - PubMed
Lildholdt 1988 {published data only}
-
- Lildholdt T, Fogstrup J, Gammelgaard N, Kortholm B, Ulsoe C. Surgical versus medical treatment of nasal polyps. Acta Oto‐Laryngologica 1988;105(1‐2):140‐3. - PubMed
Lildholdt 1989 {published data only}
-
- Lildholdt T. Surgical versus medical treatment of nasal polyps. Rhinology. Supplement 1989;8:31‐3. - PubMed
NCT01676415 {published data only}
-
- Northwestern University Feinberg School of Medicine. Corticosteroid therapy for chronic rhinosinusitis without nasal polyps (CRSsNP). http://clinicaltrials.gov/ct2/show/NCT01676415. [DOI: ]
Nores 2003 {published data only}
-
- Nores J‐M, Avan P, Bonfils P. Medical management of nasal polyposis: a study in a series of 152 consecutive patients. Rhinology 2003;41(2):97‐102. - PubMed
Ozturk 2011 {published data only}
-
- Ozturk F, Bakirtas A, Ileri F, Turktas I. Efficacy and tolerability of systemic methylprednisolone in children and adolescents with chronic rhinosinusitis: a double‐blind, placebo‐controlled randomized trial. Journal of Allergy and Clinical Immunology 2011;128(2):348‐52. - PubMed
Ragab 2006 {published data only}
-
- Ragad S, Scadding GK, Lund VJ, Saleh H. Treatment of chronic rhinosinusitis and its effects on asthma. European Respiratory Journal 2006;28(1):68‐74. - PubMed
Rasp 1997 {published data only}
-
- Rasp G, Bujia J. Treatment of nasal polyposis with systemic and local corticoids. Acta Otorrinolaringologica Espanola 1997;48(1):37‐40. - PubMed
Rasp 2000 {published data only}
-
- Rasp G, Kramer MF, Ostertag P, Kastenbauer E. A new system for the classification of ethmoid polyposis. Effect of combined local and systemic steroid therapy. Laryngo‐Rhino‐Otologie 2000;79(5):266‐72. - PubMed
Remer 2005 {published data only}
-
- Remer M, Polberg K, Obszańska B, Klatka J. Chronic sinusitis therapy with antibiotics (axetyl cefuroxym, clarithromycin) and steroid (prednisone) [Leczenie zapalenia zatok z zastosowaniem antybiotykow (aksetyl cefuroksymu, klarytromycyna) w polaczeniu ze sterydem stosowanym doustnie (prednison)]. Polski Merkuriusz Lekarski 2005;19(111):343‐4. - PubMed
Reychler 2015 {published data only}
-
- Reychler G, Colbrant C, Huart C, Guellec S, Vecellio L, Liistro G, et al. Effect of three‐drug delivery modalities on olfactory function in chronic sinusitis. Laryngoscope 2015;125(3):549‐55. - PubMed
Rupa 2010 {published data only}
Sieskiewicz 2006 {published data only}
-
- Sieskiewicz A, Olszewska E, Rogowski M, Grycz E. Preoperative corticosteroid oral therapy and intraoperative bleeding during functional endoscopic sinus surgery in patients with severe nasal polyposis: a preliminary investigation. Annals of Otology, Rhinology and Laryngology 2006;115(7):490‐4. - PubMed
Sousa 2009 {published data only}
Stevens 2001 {published data only}
-
- Stevens MH. Steroid‐dependent anosmia. Laryngoscope 2001;111(2):200‐3. - PubMed
Tuncer 2003 {published data only}
-
- Tuncer U, Soylu L, Aydogan B, Karakus F, Akcali C. The effectiveness of steroid treatment in nasal polyposis. Auris Nasus Larynx 2003;30(3):263‐8. - PubMed
van Camp 1994 {published data only}
-
- Camp C, Clement PA. Results of oral steroid treatment in nasal polyposis. Rhinology 1994;32(1):5‐9. - PubMed
References to ongoing studies
Chi 2011 {published data only}
-
- ChiCTR‐TRC‐11001323. Research on clinical efficacy of oral glucocorticoid in the treatment of eosinophilic nasal polyps and non‐eosinophilic nasal polyps. http://www.chictr.org.cn/showprojen.aspx?proj=8216.
NCT00841802 {published data only}
-
- Chronic rhinosinusitis with or without nasal polyps steroid study. http://clinicaltrials.gov/ct2/show/NCT00841802.
NCT02367118 {published data only}
-
- Prednisone in chronic rhinosinusitis without nasal polyps. http://clinicaltrials.gov/ct2/show/NCT02367118.
Additional references
Balk 2012
-
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within‐arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. - PubMed
Burton 2008
-
- Burton MJ, Harvey RJ. Idiopathic sudden sensorineural hearing loss. In: Gleeson M, Browning G, Burton MJ, Clarke R, Hibbert J, Jones NS, et al. editor(s). Scott‐Brown's Otorhinolaryngology, Head and Neck Surgery. 7th Edition. Vol. 3, London: Hodder Arnold, 2008:3577‐93.
Cho 2012
Chong 2016a
Chong 2016b
Chong 2016c
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988.
Da Silva 2006
DeMarcantonio 2011
-
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. - PubMed
Ebbens 2010
-
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. - PubMed
Ebbens 2011
-
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. - PubMed
Egger 1997
EPOS 2012
-
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. - PubMed
Fokkens 2007
-
- Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. - PubMed
Gliklich 1995
-
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hastan 2011
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. - PubMed
Head 2016a
Head 2016b
Hopkins 2016
-
- Hopkins C, Philpott C, Crowe S, Regan S, Degun A, Papachristou I, et al. Identifying the most important outcomes for systematic reviews of interventions for rhinosinusitis in adults: working with Patients, Public and Practitioners. Rhinology 2016;54(1):20‐6. - PubMed
Kern 2008
Keswani 2012
Larsen 2004
-
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. - PubMed
Martinez‐Devesa 2011
Mygind 1996
-
- Mygind N, Lildholdt T. Nasal polyps treatment: medical management. Allergy and Asthma Proceedings 1996;17:275‐82. - PubMed
Naber 1996
-
- Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8‐days' corticosteroid treatment. A prospective study. Psychoneuroendocrinology 1996;21:25‐31. - PubMed
Philpott 2015
Ragab 2004
-
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. - PubMed
Ragab 2010
-
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stanbury 1998
Tan 2011
Tomassen 2011
-
- Tomassen P, Zele T, Zhang N, Perez‐Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. - PubMed
van Drunen 2009
-
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. - PubMed
Venekamp 2014
Venekamp 2015
-
- Venekamp RP, Thompson MJ, Rovers MM. Systemic corticosteroid therapy for acute sinusitis. JAMA 2015;313(12):1258‐9. - PubMed
Zhang 2008
-
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. - PubMed
Zhang 2009
-
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

