Optimising electrogenerated chemiluminescence of quantum dots via co-reactant selection
- PMID: 27113462
- PMCID: PMC5025492
- DOI: 10.1007/s00216-016-9557-1
Optimising electrogenerated chemiluminescence of quantum dots via co-reactant selection
Abstract
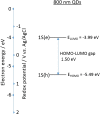
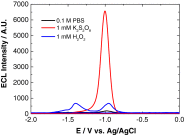
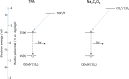
We demonstrate that for quantum dot (QD) based electrochemiluminescence (ECL), the commonly used co-reactant does not perform as effectively as potassium persulfate. By exploiting this small change in co-reactant, ECL intensity can be enhanced dramatically in a cathodic-based ECL system. However, TPA remains the preferential co-reactant-based system for anodic ECL. This phenomenon can be rationalised through the relative energy-level profiles of the QD to the co-reactant in conjunction with the applied potential range. This work highlights the importance of understanding the co-reactant pathway for optimising the application of ECL to bioanalytical analysis, in particular for near-infrared (NIR) QDs which can be utilised for analysis in blood. Graphical Abstract Optimising ECL Production Through Careful Selection of Co-Reactions Based on Energetics Involved.
Keywords: Electroanalytical methods; Electrochemiluminescence; Quantum dots.
Conflict of interest statement
The authors declare that they have no conflict of interest. Funding sources This work was supported by funding from the EU FP7 funding through the Marie Curie Reintegration Grant scheme (PIRG-2010-268236).
Figures








Similar articles
-
Cathodic Quantum Dot Facilitated Electrochemiluminescent Detection in Blood.Anal Chem. 2018 Nov 6;90(21):12944-12950. doi: 10.1021/acs.analchem.8b03572. Epub 2018 Oct 16. Anal Chem. 2018. PMID: 30280562
-
Turn-on electrochemiluminescence sensing of Cd(2+) based on CdTe quantum dots.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Dec 10;133:130-3. doi: 10.1016/j.saa.2014.05.053. Epub 2014 May 28. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24934970
-
Electrogenerated chemiluminescence resonance energy transfer between luminol and CdSe@ZnS quantum dots and its sensing application in the determination of thrombin.Anal Chem. 2014 Nov 18;86(22):11373-9. doi: 10.1021/ac5033319. Epub 2014 Nov 7. Anal Chem. 2014. PMID: 25361206
-
Recent advances of ratiometric electrochemiluminescence biosensors.J Mater Chem B. 2019 Nov 14;7(42):6469-6475. doi: 10.1039/c9tb01823a. Epub 2019 Oct 9. J Mater Chem B. 2019. PMID: 31595937 Review.
-
Electrochemiluminescence of Semiconductor Quantum Dots and Its Biosensing Applications: A Comprehensive Review.Biosensors (Basel). 2023 Jul 5;13(7):708. doi: 10.3390/bios13070708. Biosensors (Basel). 2023. PMID: 37504107 Free PMC article. Review.
Cited by
-
Ultrasensitive PCR-Free detection of whole virus genome by electrochemiluminescence.Biosens Bioelectron. 2022 Aug 1;209:114165. doi: 10.1016/j.bios.2022.114165. Epub 2022 Apr 6. Biosens Bioelectron. 2022. PMID: 35417851 Free PMC article.
References
-
- Choi JP, Bard AJ. Electrogenerated chemiluminescence (ECL) 79: reductive-oxidation ECL of tris(2,2′-bipyridine)ruthenium(II) using hydrogen peroxide as a coreactant in pH 7.5 phosphate buffer solution. Anal Chim Acta. 2005;541:141–8. doi: 10.1016/j.aca.2004.11.075. - DOI
-
- Kanoufi F, Bard AJ. Electrogenerated chemiluminescence. 65. An investigation of the oxidation of oxalate by Tris(polypyridine) ruthenium complexes and the effect of the electrochemical steps on the emission intensity. J Phys Chem B. 1999;103:10469–80. doi: 10.1021/jp992368s. - DOI
-
- McCord P, Bard AJ. Electrogenerated chemiluminescence: Part 54. Electrogenerated chemiluminescence of ruthenium(II) 4,4′-diphenyl-2,2′-bipyridine and ruthenium(II) 4,7-diphenyl-1,10-phenanthroline systems in aqueous and acetonitrile solutions. J Electroanal Chem Interfacial Electrochem. 1991;318:91–9. doi: 10.1016/0022-0728(91)85296-2. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

