Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice
- PMID: 27113500
- PMCID: PMC5102810
- DOI: 10.1016/j.biopsych.2016.03.1050
Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice
Abstract
Background: Major depressive disorder is associated with disturbed circadian rhythms. To investigate the causal relationship between mood disorders and circadian clock disruption, previous studies in animal models have employed light/dark manipulations, global mutations of clock genes, or brain area lesions. However, light can impact mood by noncircadian mechanisms; clock genes have pleiotropic, clock-independent functions; and brain lesions not only disrupt cellular circadian rhythms but also destroy cells and eliminate important neuronal connections, including light reception pathways. Thus, a definitive causal role for functioning circadian clocks in mood regulation has not been established.
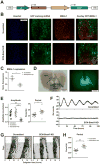
Methods: We stereotactically injected viral vectors encoding short hairpin RNA to knock down expression of the essential clock gene Bmal1 into the brain's master circadian pacemaker, the suprachiasmatic nucleus (SCN).
Results: In these SCN-specific Bmal1-knockdown (SCN-Bmal1-KD) mice, circadian rhythms were greatly attenuated in the SCN, while the mice were maintained in a standard light/dark cycle, SCN neurons remained intact, and neuronal connections were undisturbed, including photic inputs. In the learned helplessness paradigm, the SCN-Bmal1-KD mice were slower to escape, even before exposure to inescapable stress. They also spent more time immobile in the tail suspension test and less time in the lighted section of a light/dark box. The SCN-Bmal1-KD mice also showed greater weight gain, an abnormal circadian pattern of corticosterone, and an attenuated increase of corticosterone in response to stress.
Conclusions: Disrupting SCN circadian rhythms is sufficient to cause helplessness, behavioral despair, and anxiety-like behavior in mice, establishing SCN-Bmal1-KD mice as a new animal model of depression.
Keywords: Circadian clocks; Corticosterone; Depression; Learned helplessness; Mouse model; Suprachiasmatic nucleus.
Copyright © 2016 Society of Biological Psychiatry. All rights reserved.
Figures


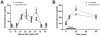

Comment in
-
Sleep and Mood: Chicken or Egg?Biol Psychiatry. 2016 Dec 1;80(11):810-811. doi: 10.1016/j.biopsych.2016.09.012. Biol Psychiatry. 2016. PMID: 27968724 No abstract available.
References
-
- Husse J, Leliavski A, Tsang AH, Oster H, Eichele G. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 2014;28:4950–4960. - PubMed
-
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

