Akt1 promotes stimuli-induced endothelial-barrier protection through FoxO-mediated tight-junction protein turnover
- PMID: 27113546
- PMCID: PMC5023469
- DOI: 10.1007/s00018-016-2232-z
Akt1 promotes stimuli-induced endothelial-barrier protection through FoxO-mediated tight-junction protein turnover
Abstract
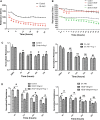
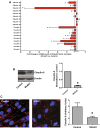
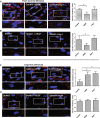
Vascular permeability regulated by the vascular endothelial growth factor (VEGF) through endothelial-barrier junctions is essential for inflammation. Mechanisms regulating vascular permeability remain elusive. Although 'Akt' and 'Src' have been implicated in the endothelial-barrier regulation, it is puzzling how both agents that protect and disrupt the endothelial-barrier activate these kinases to reciprocally regulate vascular permeability. To delineate the role of Akt1 in endothelial-barrier regulation, we created endothelial-specific, tamoxifen-inducible Akt1 knockout mice and stable ShRNA-mediated Akt1 knockdown in human microvascular endothelial cells. Akt1 loss leads to decreased basal and angiopoietin1-induced endothelial-barrier resistance, and enhanced VEGF-induced endothelial-barrier breakdown. Endothelial Akt1 deficiency resulted in enhanced VEGF-induced vascular leakage in mice ears, which was rescued upon re-expression with Adeno-myrAkt1. Furthermore, co-treatment with angiopoietin1 reversed VEGF-induced vascular leakage in an Akt1-dependent manner. Mechanistically, our study revealed that while VEGF-induced short-term vascular permeability is independent of Akt1, its recovery is reliant on Akt1 and FoxO-mediated claudin expression. Pharmacological inhibition of FoxO transcription factors rescued the defective endothelial barrier due to Akt1 deficiency. Here we provide novel insights on the endothelial-barrier protective role of VEGF in the long term and the importance of Akt1-FoxO signaling on tight-junction stabilization and prevention of vascular leakage through claudin expression.
Keywords: Akt; Angiopoietin-1; Claudin; VE-cadherin; VEGF; Vascular permeability.
Conflict of interest statement
Authors declare that there are no financial or conflicts of interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

