Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica
- PMID: 27113605
- PMCID: PMC5013123
- DOI: 10.1136/jnnp-2015-312601
Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica
Abstract
Objective: Antibodies to cell surface central nervous system proteins help to diagnose conditions which often respond to immunotherapies. The assessment of antibody assays needs to reflect their clinical utility. We report the results of a multicentre study of aquaporin (AQP) 4 antibody (AQP4-Ab) assays in neuromyelitis optica spectrum disorders (NMOSD).
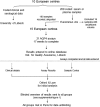
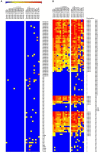
Methods: Coded samples from patients with neuromyelitis optica (NMO) or NMOSD (101) and controls (92) were tested at 15 European diagnostic centres using 21 assays including live (n=3) or fixed cell-based assays (n=10), flow cytometry (n=4), immunohistochemistry (n=3) and ELISA (n=1).
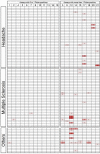
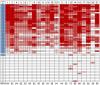
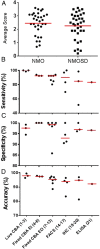
Results: Results of tests on 92 controls identified 12assays as highly specific (0-1 false-positive results). 32 samples from 50 (64%) NMO sera and 34 from 51 (67%) NMOSD sera were positive on at least two of the 12 highly specific assays, leaving 35 patients with seronegative NMO/spectrum disorder (SD). On the basis of a combination of clinical phenotype and the highly specific assays, 66 AQP4-Ab seropositive samples were used to establish the sensitivities (51.5-100%) of all 21 assays. The specificities (85.8-100%) were based on 92 control samples and 35 seronegative NMO/SD patient samples.
Conclusions: The cell-based assays were most sensitive and specific overall, but immunohistochemistry or flow cytometry could be equally accurate in specialist centres. Since patients with AQP4-Ab negative NMO/SD require different management, the use of both appropriate control samples and defined seronegative NMOSD samples is essential to evaluate these assays in a clinically meaningful way. The process described here can be applied to the evaluation of other antibody assays in the newly evolving field of autoimmune neurology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
