Vapocoolants (cold spray) for pain treatment during intravenous cannulation
- PMID: 27113639
- PMCID: PMC8666144
- DOI: 10.1002/14651858.CD009484.pub2
Vapocoolants (cold spray) for pain treatment during intravenous cannulation
Abstract
Background: Intravenous cannulation is a painful procedure that can provoke anxiety and stress. Injecting local anaesthetic can provide analgesia at the time of cannulation, but it is a painful procedure. Topical anaesthetic creams take between 30 and 90 minutes to produce an effect. A quicker acting analgesic allows more timely investigation and treatment. Vapocoolants have been used in this setting, but studies have reported mixed results.
Objectives: To determine effects of vapocoolants on pain associated with intravenous cannulation in adults and children. To explore variables that might affect the performance of vapocoolants, including time required for application, distance from the skin when applied and time to cannulation. To look at adverse effects associated with the use of vapocoolants.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Latin American Caribbean Health Sciences Literature (LILACS), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Institute for Scientific Information (ISI) Web of Science and the http://clinicaltrials.gov/, http://www.controlled-trials.com/ and http://www.trialscentral.org/ databases to 1 May 2015. We applied no language restrictions. We also scanned the reference lists of included papers.
Selection criteria: We included all blinded and unblinded randomized controlled trials (RTCs) comparing any vapocoolant with placebo or control to reduce pain during intravenous cannulation in adults and children.
Data collection and analysis: Three review authors independently assessed trial quality and extracted data, contacted study authors for additional information and assessed included studies for risk of bias. We collected and analysed data for the primary outcome of pain during cannulation, and for the secondary outcomes of pain associated with application of the vapocoolant, first attempt success rate of intravenous cannulation, adverse events and participant satisfaction. We performed subgroup analyses for the primary outcome to examine differences based on age of participant, type of vapocoolant used, application time of vapocoolant and clinical situation (emergency vs elective). We used random-effects model meta-analysis in RevMan 5.3 and assessed heterogeneity between trial results by examining forest plots and calculating the I(2) statistic.
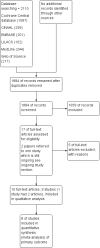
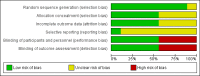
Main results: We found nine suitable studies of 1070 participants and included them in the qualitative analyses. We included eight studies of 848 participants in the meta-analysis for the primary outcome (pain during intravenous cannulation). Use of vapocoolants resulted in a reduction in pain scores as measured by a linear 100 mm visual analogue scale (VAS 100) compared with controls (difference between means -12.5 mm, 95% confidence interval (CI) -18.7 to -6.4 mm; moderate-quality evidence). We could not include in the meta-analysis one study, which showed no effects of the intervention.Use of vapocoolants resulted in increased pain scores at the time of application as measured by a VAS 100 compared with controls (difference between means 6.3 mm, 95% CI 2.2 to 10.3 mm; four studies, 461 participants; high-quality evidence) and led to no difference in first attempt success compared with controls (risk ratio (RR) 1.00, 95% CI 0.94 to 1.06; six studies, 812 participants; moderate-quality evidence). We documented eight minor adverse events reported in 279 vapocoolant participants (risk difference (RD) 0.03, 95% CI 0 to 0.05; five studies, 551 participants; low quality-evidence).The overall risk of bias of individual studies ranged from low to high, with high risk of bias for performance and detection bias in four studies. Sensitivity analysis showed that exclusion of studies at high or unclear risk of bias did not materially alter the results of this review.
Authors' conclusions: Moderate-quality evidence indicates that use of a vapocoolant immediately before intravenous cannulation reduces pain during the procedure. Use of vapocoolant does not increase the difficulty of cannulation nor cause serious adverse effects but is associated with mild discomfort during application.
Conflict of interest statement
Rebecca J Griffith: none known.
Vanessa Jordan. none known.
David Herd: none known.
Peter W Reed: none known.
Stuart R Dalziel: none known.
Figures

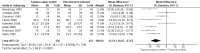














Comment in
-
Effect of Vapocoolant on Pain During Peripheral Intravenous Cannulation.Ann Emerg Med. 2016 Nov;68(5):586-588. doi: 10.1016/j.annemergmed.2016.06.001. Epub 2016 Jun 30. Ann Emerg Med. 2016. PMID: 27374950 No abstract available.
References
References to studies included in this review
Armstrong 1990 {published data only}
-
- Armstrong P, Young C, McKeown D. Ethyl chloride and venepuncture pain: a comparison with intradermal lidocaine. Canadian Journal of Anaesthesia 1990; Vol. 37, issue 6:656‐8. [PUBMED: 2208537] - PubMed
Costello 2006 {published data only}
-
- Costello M, Ramundo M, Christopher NC, Powell KR. Ethyl vinyl chloride vapocoolant spray fails to decrease pain associated with intravenous cannulation in children. Clinical Pediatrics 2006;45(7):628‐32. [PUBMED: 16928840] - PubMed
-
- Costello M, Ramundo M, Christopher NC, Powell KR. Lack of effect of etiryl vinyl chloride vapocoolant spray on decreasing pain associated with IV cannulation in children: a brief report. Pediatric Research 2002;51(4):43A.
Crecelius 1999 {published data only}
Farion 2008 {published data only}
Hartstein 2008 {published data only}
-
- Hartstein BH, Barry JD. Mitigation of pain during intravenous catheter placement using a topical skin coolant in the emergency department. Emergency Medicine Journal 2008; Vol. 25, issue 5:257‐61. [PUBMED: 18434455] - PubMed
Hijazi 2009 {published data only}
Ramsook 2001 {published data only}
-
- Ramsook C, Kozinetz CA, Moro‐Sutherland D. Efficacy of ethyl chloride as a local anesthetic for venipuncture and intravenous cannula insertion in a pediatric emergency department. Pediatric Emergency Care 2001; Vol. 17, issue 5:341‐3. [PUBMED: 11673710] - PubMed
Robinson 2007 {published data only}
-
- Robinson PA, Carr S, Pearson S, Frampton C. Lignocaine is a better analgesic than either ethyl chloride or nitrous oxide for peripheral intravenous cannulation. Emergency Medicine Australasia 2007; Vol. 19, issue 5:427‐32. [PUBMED: 17919215] - PubMed
References to studies excluded from this review
Baelen 1994 {published data only}
-
- Baelen E, Dalmas S, Ducloux B, Scherpereel P. Cryoanaesthesia with a freon spray for venipuncture in children. Annales Francaises D'Anesthesie et de Reanimation 1994; Vol. 13, issue 1:6‐9. [PUBMED: 8092581] - PubMed
Baxter 2009 {published data only}
-
- Baxter AL, Leong T, Mathew B. External thermomechanical stimulation versus vapocoolant for adult venipuncture pain: pilot data on a novel device. The Clinical Journal of Pain 2009; Vol. 25, issue 8:705‐10. [PUBMED: 19920721] - PubMed
Kelly 2008 {published data only}
-
- Kelly JS. Painless vascular cannulation: ethyl chloride, vapocoolants and cryoanalgesics. Journal of the Royal College of Physicians of Edinburgh 2008; Vol. 38:232‐3.
Lunoe 2015 {published data only}
Soueid 2007 {published data only}
-
- Soueid A, Richard B. Ethyl chloride as a cryoanalgesic in pediatrics for venipuncture. Pediatric Emergency Care 2007; Vol. 23:380‐3. [PUBMED: 17572521] - PubMed
References to ongoing studies
Mace 2014 {published data only}
-
- Mace SE. Can we decrease the pain of peripheral intravenous line placement in adults by the use of vapocoolant spray? Preliminary results of a prospective, randomized, blinded, placebo‐controlled trial. Academic Emergency Medicine. Blackwell Publishing Ltd, 2014, issue 5 Suppl 1:S20.
-
- Mace SE. Prospective, randomized, blinded, placebo‐controlled trial of the use of vapocoolant spray to decrease the pain of peripheral intravenous line placement in adults in the emergency department: preliminary results. Annals of Emergency Medicine. Mosby Inc., 2014, issue 4 Suppl 1:S128.
Additional references
Altman 2000
Berlin 1997
-
- Berlin JA. Does blinding of readers affect the results of meta‐analyses?. Lancet 1997;350:185‐6. [PUBMED: 9250191] - PubMed
Burke 1999
-
- Burke D, Mogyoros I, Vagg R, Kiernan MC. Temperature dependence of excitability indices of human cutaneous afferents. Muscle and Nerve 1999;22(1):51‐60. [PUBMED: 9883857 ] - PubMed
Guyatt 2008
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kennedy 1999
-
- Kennedy RM, Luhmann JD. The "ouchless emergency department". Getting closer: advances in decreasing distress during painful procedures in the emergency department. Pediatric Clinics of North America 1999;46(6):1215‐47. [PUBMED: 10629683] - PubMed
Lander 2006
Mawhorter 2004
-
- Mawhorter S, Daugherty L, Ford A, Hughes R, Metzger D, Easley K. Topical vapocoolant qUickly and effectively reduces vacclne‐associated pain: results of a randomized, single‐blinded, placebo‐controlled study. Journal of Travel Medicine 2004;11:262‐72. - PubMed
Moher 2009
Moore 2009
-
- Moore A, Straube S, McQuay H. Minimising pain during intravenous cannulation. BMJ 2009;338:422‐3. [PUBMED: 19208702] - PubMed
RevMan 5.3 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shah 2009
-
- Shah V, Taddio A, Rieder MJ, for the HELPinKIDS Team. Effectiveness and tolerability of pharmacologic and combined interventions for reducing injection pain during routine childhood immunizations: systematic review and meta‐analyses. Clinical Therapeutics 2009;31(Suppl B):S104‐51. - PubMed
Skarbek‐Borowska 2006
-
- Skarbek‐Borowska S, Becker BM, Lovgren K, Bates A, Minugh PA. Brief focal ultrasound with topical anesthetic decreases the pain of intravenous placement in children. Pediatric Emergency Care 2006;22(5):339‐45. [PUBMED: 16714961 ] - PubMed
Todd 1996
-
- Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Annals of Emergency Medicine 1996;27:485‐9. - PubMed
Uman 2006
Zempsky 2008a
-
- Zempsky WT. Pharmacological approaches for reducing venous access pain in children. Pediatrics 2008;122:S140‐53. [PUBMED: 18978008 ] - PubMed
Zempsky 2008b
-
- Zempsky WT, Bean‐Lijewski J, Kauffman RE, Koh JL, Malviya SV, Rose JB, et al. Needle‐free powder lidocaine delivery system provides rapid effective analgesia for venipuncture or cannulation pain in children: randomized, double‐blind comparison of venipuncture and venous cannulation pain after fast‐onset needle‐free powder lidocaine or placebo treatment trial. Pediatrics 2008;121(5):979‐87. [PUBMED: 18450903 ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

