The paracaspase MALT1 mediates CARD14-induced signaling in keratinocytes
- PMID: 27113748
- PMCID: PMC5278603
- DOI: 10.15252/embr.201642109
The paracaspase MALT1 mediates CARD14-induced signaling in keratinocytes
Abstract
Mutations in CARD14 have recently been linked to psoriasis susceptibility. CARD14 is an epidermal regulator of NF-κB activation. However, the ability of CARD14 to activate other signaling pathways as well as the biochemical mechanisms that mediate and regulate its function remain to be determined. Here, we report that in addition to NF-κB signaling, CARD14 activates p38 and JNK MAP kinase pathways, all of which are dependent on the paracaspase MALT1. Mechanistically, we demonstrate that CARD14 physically interacts with paracaspase MALT1 and activates MALT1 proteolytic activity and inflammatory gene expression, which are enhanced by psoriasis-associated CARD14 mutations. Moreover, we show that MALT1 deficiency or pharmacological inhibition of MALT1 catalytic activity inhibits pathogenic mutant CARD14-induced cytokine and chemokine expression in human primary keratinocytes. Collectively, our findings demonstrate a novel role for MALT1 in CARD14-induced signaling and indicate MALT1 as a valuable therapeutic target in psoriasis.
Keywords: CARD14 signaling pathway; MALT1; inflammation; proteolytic activity; psoriasis.
© 2016 The Authors.
Figures

NF‐κB reporter gene expression.
IL‐8 secretion.
Immunoblotting for c‐Jun, phospho‐c‐Jun, JNK, phospho‐JNK, p38, and phospho‐p38 as indicated. Actin was used as a loading control. Data are representative of two independent experiments.


MALT1 interacts with CARD14sh in a BCL10‐dependent manner. HEK293T cells were transfected with FLAG‐CARD14sh, Myc‐MALT1, and FLAG‐BCL10 as indicated. Twenty‐four hours later, MALT1 was immunoprecipitated (IP) from cell lysates with anti‐MALT1. CARD14sh and BCL10 co‐immunoprecipitation with MALT1 was detected by immunoblotting with anti‐FLAG. Immunoprecipitated MALT1 was detected by immunoblotting with anti‐myc. The closed arrowhead shows the position of CARD14, and the double arrowhead shows the position of BCL10. The asterisk indicates a non‐specific band. Total expression levels of transfected proteins are shown by immunoblotting of a fraction of the cell lysates with the indicated antibodies (bottom panel).
MALT1 co‐expression potentiates CARD14sh‐induced JNK and p38 MAP kinase activation. HEK293T cells were transfected with the indicated concentrations of FLAG‐tagged CARD14sh, with or without MALT1 (30 ng). Transfection with FLAG‐TRAF6 and FLAG‐CARD11(L232LI) was used as a positive control. c‐Jun accumulation, JNK and p38 phosphorylation were analyzed by immunoblotting with the antibodies indicated. Actin was used as a loading control.
MALT1 co‐expression potentiates NF‐κB activation by pathogenic CARD14 mutants. HEK293T were transfected with NF‐κB reporter plasmid and wild‐type (WT) FLAG‐CARD14 or the indicated psoriasis‐associated CARD14 mutants (15 ng), with or without MALT1 (15 ng). Luciferase activity in cell lysates was measured after 24 h. Values are the mean of triplicates ± SE. Significance levels: **P < 0.01 and ***P < 0.001 by Student's t‐test. Expression of transfected proteins was verified by Western blotting (bottom panel).
CARD14(E138A) mutation enhances MALT1 binding. HEK293T cells were transfected with wild‐type (WT) FLAG‐CARD14sh or the indicated CARD14sh mutants and Myc‐MALT1. Twenty‐four hours later, MALT1 was immunoprecipitated (IP) from cell lysates with anti‐MALT1 and CARD14 co‐immunoprecipitation was detected by immunoblotting with anti‐FLAG. Immunoprecipitated MALT1 was detected by immunoblotting with anti‐MALT1. The asterisk indicates a non‐specific band. Immunoprecipitation with a non‐relevant antibody (rIgG) was used as a negative control (first lane). Total expression levels of transfected proteins are shown by immunoblotting of total cell lysates (bottom panel). Data are representative of two independent experiments.

Effect of BCL10 knockdown on CARD14sh‐induced NF‐κB signaling. HEK293T cells were transfected with scrambled (scr) or BCL10 siRNA. Cells were replated and transfected 48 h later with NF‐κB reporter plasmid and FLAG‐CARD14sh (20 ng). Luciferase activity in cell lysates was measured after 24 h. BCL10 knockdown was verified by immunoblotting (inset). Values are the mean of triplicates ± SE. Significance levels: ***P < 0.001 by Student's t‐test.
Comparison of the ability of different CARD family proteins to form a complex with MALT1 and BCL10. HEK293T cells were transfected with Myc‐MALT1, FLAG‐BCL10, FLAG‐CARD14, FLAG‐CARD14sh, FLAG‐CARD9, or FLAG‐CARD11(L232LI) as indicated. After 48 h, cell lysates were immunoprecipitated (IP) with anti‐MALT1 and co‐immunoprecipitation of specific CARD family proteins and BCL10 was detected by immunoblotting with anti‐FLAG. Immunoprecipitation with a non‐relevant antibody (rIgG) was used as a negative control (last lane). Total expression levels of transfected proteins are shown by immunoblotting of a fraction of the cell lysates with the indicated antibodies (bottom panel). Data are representative of two independent experiments.

MALT1 expression is essential for CARD14sh‐induced NF‐κB activation. MALT1‐deficient HEK293T cells were transfected with NF‐κB reporter plasmid and the indicated expression plasmids. Luciferase activity in cell lysates was measured 24 h later. CARD11(L232LI) and TRAF6 transfections were used as MALT1‐dependent and MALT1‐independent controls, respectively. Expression of the transfected plasmids was verified by immunoblotting (lower panel).
MALT1 expression is essential for CARD14sh‐induced JNK activation. MALT1‐deficient HEK293T cells were transfected with FLAG‐CARD14sh (30 ng) either alone or together with either wild‐type (MALT1WT) or catalytically inactive MALT1 (MALT1C/A) as indicated. c‐Jun accumulation and JNK phosphorylation were analyzed by immunoblotting with the antibodies indicated. Actin was used as a loading control.
MALT1 deficiency inhibits CARD14‐induced gene expression in HaCaT keratinocytes. HaCaT cells were transfected with scrambled (scr) or MALT1‐targeting siRNA prior to doxycycline‐induced CARD14 expression as described in Materials and Methods. IL‐8 and MCP‐1 concentration in the cell supernatants was measured 8 h (IL‐8) or 24 h (MCP‐1) later by ELISA. Doxycycline‐induced CARD14 expression and MALT1 knockdown were verified by immunoblotting (right panel). Data are representative of two independent experiments.
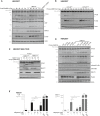
CARD14 signaling induces CYLD and A20 cleavage. HEK293T cells were transfected with different concentrations of FLAG‐CARD14, with or without MALT1 as indicated. CARD11(L232LI) transfection was used as a positive control. Endogenous CYLD and A20 processing was analyzed by immunoblotting. CYLD and A20 cleavage fragments are indicated by an arrow, the asterisk indicates a non‐specific band.
CARD14 signaling induces CYLD cleavage after R324. HEK293T cells were transfected with different concentrations of FLAG‐CARD14sh and either wild‐type (CYLDWT) or non‐cleavable CYLD (CYLDR324A). CYLD processing was analyzed by immunoblotting. The CYLD cleavage fragment is indicated by an arrow.
Expression of catalytically active MALT1 is necessary for CARD14‐induced CYLD cleavage. MALT1‐deficient HEK293T cells, in which MALT1 expression was restored by transfection with different concentrations of either wild‐type (MALT1WT) or catalytically inactive MALT1 (MALT1C/A), were co‐transfected with FLAG‐CARD14sh and CYLD as indicated. CYLD processing was analyzed by immunoblotting. The CYLD cleavage fragment is indicated by an arrow.
Psoriasis‐associated CARD14 mutation enhances activation of MALT1 proteolytic activity. HEK293T cells were transfected with wild‐type (WT) FLAG‐CARD14 or different psoriasis‐associated CARD14 mutants (20 ng), with or without MALT1 (20 ng) as indicated. Endogenous CYLD and A20 processing, as well as IκBα phosphorylation, was analyzed by immunoblotting. The CYLD and A20 cleavage fragments are indicated by an arrow.
Pharmacological inhibition of MALT1 proteolytic activity prevents CARD14‐induced gene expression in HaCaT keratinocytes. CARD14 expression was induced by doxycycline (dox) treatment in the absence or presence of different concentrations of mepazine as indicated. The next morning, cell culture medium was refreshed and IL‐8 and MCP‐1 concentration in the cell supernatant was measured 8 h later by ELISA. TNF stimulation (MALT1‐independent) was used as a negative control. Values are the mean of triplicates ± SE. Data are representative of two independent experiments. Significance levels: *P < 0.05, **P < 0.001 by Student's t‐test.
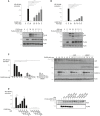
- A, B
CYLD and A20 inhibit CARD14sh‐induced NF‐κB activation. HEK293T cells were transfected with NF‐κB reporter plasmid and FLAG‐tagged CARD14sh (30 ng), with or without the indicated concentrations of CYLD (A) or A20 (B). Luciferase activity in cell lysates was measured 24 h later. Protein expression of transfected plasmids was verified by Western blot and is shown in the accompanying panels. Closed arrowheads indicate full‐length A20 and CYLD; arrows indicate cleaved A20 and CYLD. Data are representative of three independent experiments.
- C
A20 and ABIN‐1 inhibit CARD14‐induced NF‐κB activation. HEK293T cells were transfected with NF‐κB reporter plasmid, A20 (3 ng) or ABIN1 (15 ng) and the indicated concentrations of FLAG‐tagged CARD14sh. Luciferase activity in cell lysates was measured 24 h later. Protein expression of transfected plasmids was verified by Western blot and is shown in the accompanying panels. Data are representative of three independent experiments.
- D
A20 inhibits CARD14‐induced NF‐κB activation through its ZnF domains. HEK293T cells were transfected with NF‐κB reporter plasmid, FLAG‐CARD14sh (20 ng) and wild‐type (WT) or mutant A20 expression plasmids (3 ng) as indicated. Luciferase activity in cell lysates was measured after 24 h. CARD14 and A20 expression was measured by immunoblotting (right panel). Data are representative of two independent experiments.
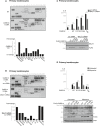
CARD14(E138A) induces cytokine and chemokine expression in human primary keratinocytes. Cells were transfected with empty vector (EV) or CARD14(E138A) as indicated. Twenty‐four hours later, the secretion of 36 different human cytokines, chemokines, and acute‐phase proteins was analyzed via a multiplex antibody array. Quantification was done using ImageJ software and is expressed as relative CARD14/EV values for a selected number of proteins (bottom panel).
MALT1 deficiency inhibits CARD14(E138A)‐induced cytokine and chemokine expression in human primary keratinocytes. Cells were transfected with scrambled (scr) or MALT1‐targeting siRNA as indicated, 48 h later followed by transfection with CARD14(E138A). After 24 h, cytokine and chemokine expression was analyzed and is expressed as described in (A).
Pathogenic CARD14 mutation enhances MALT1‐dependent CARD14‐induced IL‐8 expression in human primary keratinocytes. Cells were transfected with scrambled (scr) or MALT1‐targeting siRNA as indicated, 48 h later followed by transfection with CARD14 wild‐type (WT), CARD14(G117S), or CARD14(E138A) as indicated. TNF treatment was used as a control. After 24 h, IL‐8 concentration in the cell supernatant was analyzed via ELISA. Expression levels of MALT1 and CARD14 variants were verified by immunoblotting (bottom panel).
Pharmacological inhibition of MALT1 proteolytic activity inhibits CARD14‐induced IL‐8 production and A20 processing in human primary keratinocytes. Cells were transfected with different CARD14 variants in the presence or absence of mepazine as indicated. TNF treatment was used as a control. Twenty‐four hours later, IL‐8 levels in the cell supernatant were measured by ELISA. Expression of different CARD14 variants and CARD14‐induced processing of endogenous A20 were analyzed by immunoblotting. A20 cleavage fragment is indicated by an arrow. Data are representative of two independent experiments.

- A, B
Longer exposure of the multiplex antibody array shown in Fig 6.
- C
Position of all 36 human cytokines, chemokines, and acute‐phase proteins on the multiplex antibody array.
References
-
- Albanesi C, De Pita O, Girolomoni G (2007) Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol 25: 581–588 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

