Treatment Response and Long-Term Outcome of Peginterferon α and Ribavirin Therapy in Korean Patients with Chronic Hepatitis C
- PMID: 27114417
- PMCID: PMC5003206
- DOI: 10.5009/gnl15360
Treatment Response and Long-Term Outcome of Peginterferon α and Ribavirin Therapy in Korean Patients with Chronic Hepatitis C
Abstract
Background/aims: Peginterferon plus ribavirin remains a standard therapy for patients with chronic hepatitis C (CHC) in Korea. We investigated the efficacy and long-term outcome of peginterferon and ribavirin therapy in Korean patients with CHC, particularly in relation to the stage of liver fibrosis.
Methods: The incidence of sustained virological response (SVR), hepatic decompensation, hepatocellular carcinoma, and liver-related death was analyzed in 304 patients with CHC; the patients were followed up for a median of 54 months.
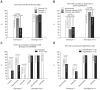
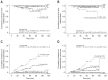
Results: Among patients with HCV genotype 1, the SVR rate was 36.7% (18/49) and 67% (69/103) for patients with and without cirrhosis, respectively (p<0.001). For patients with non-1 HCV genotypes, the SVR rates were 86.0% (37/43) in cirrhotic patients and 86.2% (94/109) in noncirrhotic patients. SVR significantly reduced the risk of liverrelated death, hepatic decompensation, and hepatocellular carcinoma, which had hazard ratios of 0.27, 0.16, and 0.22, respectively (all p<0.05). However, despite the SVR rate, patients with advanced fibrosis were still at risk of developing liver-related complications.
Conclusions: A relatively high SVR rate was achieved by peginterferon plus ribavirin therapy in Korean patients with CHC, which improved their long-term outcomes. However, all CHC patients with advanced hepatic fibrosis should receive close follow-up observations, even after successful antiviral treatment.
Keywords: Hepatitis C; Hepatitis C virus clinical trials; Hepatitis C virus treatment; Viral hepatitis; clinical.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

