PilZ Domain Protein FlgZ Mediates Cyclic Di-GMP-Dependent Swarming Motility Control in Pseudomonas aeruginosa
- PMID: 27114465
- PMCID: PMC4907108
- DOI: 10.1128/JB.00196-16
PilZ Domain Protein FlgZ Mediates Cyclic Di-GMP-Dependent Swarming Motility Control in Pseudomonas aeruginosa
Abstract
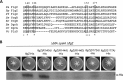
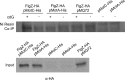
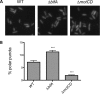
The second messenger cyclic diguanylate (c-di-GMP) is an important regulator of motility in many bacterial species. In Pseudomonas aeruginosa, elevated levels of c-di-GMP promote biofilm formation and repress flagellum-driven swarming motility. The rotation of P. aeruginosa's polar flagellum is controlled by two distinct stator complexes, MotAB, which cannot support swarming motility, and MotCD, which promotes swarming motility. Here we show that when c-di-GMP levels are elevated, swarming motility is repressed by the PilZ domain-containing protein FlgZ and by Pel polysaccharide production. We demonstrate that FlgZ interacts specifically with the motility-promoting stator protein MotC in a c-di-GMP-dependent manner and that a functional green fluorescent protein (GFP)-FlgZ fusion protein shows significantly reduced polar localization in a strain lacking the MotCD stator. Our results establish FlgZ as a c-di-GMP receptor affecting swarming motility by P. aeruginosa and support a model wherein c-di-GMP-bound FlgZ impedes motility via its interaction with the MotCD stator.
Importance: The regulation of surface-associated motility plays an important role in bacterial surface colonization and biofilm formation. c-di-GMP signaling is a widespread means of controlling bacterial motility, and yet the mechanism whereby this signal controls surface-associated motility in P. aeruginosa remains poorly understood. Here we identify a PilZ domain-containing c-di-GMP effector protein that contributes to c-di-GMP-mediated repression of swarming motility by P. aeruginosa We provide evidence that this effector, FlgZ, impacts swarming motility via its interactions with flagellar stator protein MotC. Thus, we propose a new mechanism for c-di-GMP-mediated regulation of motility for a bacterium with two flagellar stator sets, increasing our understanding of surface-associated behaviors, a key prerequisite to identifying ways to control the formation of biofilm communities.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

