Targeting lymphoma with precision using semisynthetic anti-idiotype peptibodies
- PMID: 27114517
- PMCID: PMC4868466
- DOI: 10.1073/pnas.1603335113
Targeting lymphoma with precision using semisynthetic anti-idiotype peptibodies
Abstract
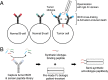
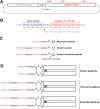
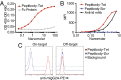
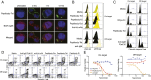
B-cell lymphomas express a functionally active and truly tumor-specific cell-surface product, the variable region of the B-cell receptor (BCR), otherwise known as idiotype. The tumor idiotype differs, however, from patient to patient, making it a technical challenge to exploit for therapy. We have developed a method of targeting idiotype by using a semisynthetic personalized therapeutic that is more practical to produce on a patient-by-patient basis than monoclonal antibodies. In this method, a small peptide with affinity for a tumor idiotype is identified by screening a library, chemically synthesized, and then affixed to the amino terminus of a premade IgG Fc protein. We demonstrate that the resultant semisynthetic anti-idiotype peptibodies kill tumor cells in vitro with specificity, trigger tumor cell phagocytosis by macrophages, and efficiently clear human lymphoma in a murine xenograft model. This method could be used to target tumor with true precision on a personalized basis.
Keywords: idiotype; lymphoma; peptibody; precision medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Specific Targeting of Lymphoma Cells Using Semisynthetic Anti-Idiotype Shark Antibodies.Front Immunol. 2020 Nov 26;11:560244. doi: 10.3389/fimmu.2020.560244. eCollection 2020. Front Immunol. 2020. PMID: 33324393 Free PMC article.
-
Antibody responses in melanoma patients immunized with an anti-idiotype antibody mimicking disialoganglioside GD2.Clin Cancer Res. 1998 May;4(5):1117-24. Clin Cancer Res. 1998. PMID: 9607568 Clinical Trial.
-
Generation of anti-idiotype scFv for pharmacokinetic measurement in lymphoma patients treated with chimera anti-CD22 antibody SM03.PLoS One. 2014 May 9;9(5):e96697. doi: 10.1371/journal.pone.0096697. eCollection 2014. PLoS One. 2014. PMID: 24816427 Free PMC article.
-
Idiotype vaccines for lymphoma therapy.Expert Rev Vaccines. 2011 Jun;10(6):801-9. doi: 10.1586/erv.11.44. Expert Rev Vaccines. 2011. PMID: 21692701 Review.
-
Therapy of lymphoma directed at idiotypes.J Natl Cancer Inst Monogr. 1990;(10):61-8. J Natl Cancer Inst Monogr. 1990. PMID: 1693283 Review.
Cited by
-
Spatial Transcriptomics Resolve an Emphysema-Specific Lymphoid Follicle B Cell Signature in Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2024 Jan 1;209(1):48-58. doi: 10.1164/rccm.202303-0507LE. Am J Respir Crit Care Med. 2024. PMID: 37934672 Free PMC article.
-
Specific Targeting of Lymphoma Cells Using Semisynthetic Anti-Idiotype Shark Antibodies.Front Immunol. 2020 Nov 26;11:560244. doi: 10.3389/fimmu.2020.560244. eCollection 2020. Front Immunol. 2020. PMID: 33324393 Free PMC article.
-
Shark IgNAR: The Next Broad Application Antibody in Clinical Diagnoses and Tumor Therapies?Mar Drugs. 2023 Sep 16;21(9):496. doi: 10.3390/md21090496. Mar Drugs. 2023. PMID: 37755109 Free PMC article. Review.
-
Sactipeptide Engineering by Probing the Substrate Tolerance of a Thioether-Bond-Forming Sactisynthase.Angew Chem Int Ed Engl. 2022 Nov 7;61(45):e202210883. doi: 10.1002/anie.202210883. Epub 2022 Oct 12. Angew Chem Int Ed Engl. 2022. PMID: 36049110 Free PMC article.
-
Identification of the atypically modified autoantigen Ars2 as the target of B-cell receptors from activated B-cell-type diffuse large B-cell lymphoma.Haematologica. 2021 Aug 1;106(8):2224-2232. doi: 10.3324/haematol.2019.241653. Haematologica. 2021. PMID: 32675228 Free PMC article.
References
-
- Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380(9844):848–857. - PubMed
-
- Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol. 2011;29(14):1885–1892. - PubMed
-
- Morrison VA. Immunosuppression associated with novel chemotherapy agents and monoclonal antibodies. Clin Infect Dis. 2014;59(Suppl 5):S360–S364. - PubMed
-
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. - PubMed
-
- Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375(6529):334–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

