Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus
- PMID: 27114552
- PMCID: PMC4868487
- DOI: 10.1073/pnas.1522014113
Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus
Abstract
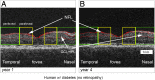
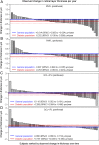
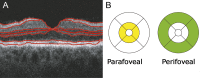
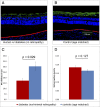
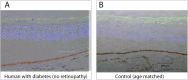
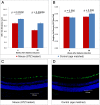
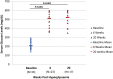
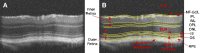
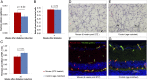
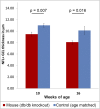
Diabetic retinopathy (DR) has long been recognized as a microvasculopathy, but retinal diabetic neuropathy (RDN), characterized by inner retinal neurodegeneration, also occurs in people with diabetes mellitus (DM). We report that in 45 people with DM and no to minimal DR there was significant, progressive loss of the nerve fiber layer (NFL) (0.25 μm/y) and the ganglion cell (GC)/inner plexiform layer (0.29 μm/y) on optical coherence tomography analysis (OCT) over a 4-y period, independent of glycated hemoglobin, age, and sex. The NFL was significantly thinner (17.3 μm) in the eyes of six donors with DM than in the eyes of six similarly aged control donors (30.4 μm), although retinal capillary density did not differ in the two groups. We confirmed significant, progressive inner retinal thinning in streptozotocin-induced "type 1" and B6.BKS(D)-Lepr(db)/J "type 2" diabetic mouse models on OCT; immunohistochemistry in type 1 mice showed GC loss but no difference in pericyte density or acellular capillaries. The results suggest that RDN may precede the established clinical and morphometric vascular changes caused by DM and represent a paradigm shift in our understanding of ocular diabetic complications.
Keywords: diabetes; diabetic retinopathy; neurodegeneration; optical coherence tomography; retina.
Conflict of interest statement
Conflict of interest statement: M.D.A. has direct equity ownership of and receives income from IDx LLC, a company that has licensed inventions from the University of Iowa, on which the Iowa Reference Algorithms used in this study are based. M.D.A. and M.S. are inventors on patents assigned to the University of Iowa and licensed by IDx LLC.
Figures

References
-
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. - PubMed
-
- Antonetti DA, et al. JDRF Diabetic Retinopathy Center Group Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. - PubMed
-
- Realini T, Lai MQ, Barber L. Impact of diabetes on glaucoma screening using frequency-doubling perimetry. Ophthalmology. 2004;111(11):2133–2136. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

