Characterization of Drosophila CMP-sialic acid synthetase activity reveals unusual enzymatic properties
- PMID: 27114558
- PMCID: PMC5086458
- DOI: 10.1042/BCJ20160347
Characterization of Drosophila CMP-sialic acid synthetase activity reveals unusual enzymatic properties
Abstract
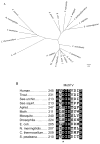
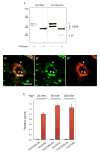
CMP-sialic acid synthetase (CSAS) is a key enzyme of the sialylation pathway. CSAS produces the activated sugar donor, CMP-sialic acid, which serves as a substrate for sialyltransferases to modify glycan termini with sialic acid. Unlike other animal CSASs that normally localize in the nucleus, Drosophila melanogaster CSAS (DmCSAS) localizes in the cell secretory compartment, predominantly in the Golgi, which suggests that this enzyme has properties distinct from those of its vertebrate counterparts. To test this hypothesis, we purified recombinant DmCSAS and characterized its activity in vitro Our experiments revealed several unique features of this enzyme. DmCSAS displays specificity for N-acetylneuraminic acid as a substrate, shows preference for lower pH and can function with a broad range of metal cofactors. When tested at a pH corresponding to the Golgi compartment, the enzyme showed significant activity with several metal cations, including Zn(2+), Fe(2+), Co(2+) and Mn(2+), whereas the activity with Mg(2+) was found to be low. Protein sequence analysis and site-specific mutagenesis identified an aspartic acid residue that is necessary for enzymatic activity and predicted to be involved in co-ordinating a metal cofactor. DmCSAS enzymatic activity was found to be essential in vivo for rescuing the phenotype of DmCSAS mutants. Finally, our experiments revealed a steep dependence of the enzymatic activity on temperature. Taken together, our results indicate that DmCSAS underwent evolutionary adaptation to pH and ionic environment different from that of counterpart synthetases in vertebrates. Our data also suggest that environmental temperatures can regulate Drosophila sialylation, thus modulating neural transmission.
Keywords: CMP-sialic acid synthetase; Drosophila glycosylation; enzyme evolution; sialic acid; sialylation.
© 2016 The Author(s). published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
CMP-sialic acid synthetase in Drosophila requires N-glycosylation of a noncanonical site.J Biol Chem. 2025 Jun;301(6):108483. doi: 10.1016/j.jbc.2025.108483. Epub 2025 Apr 7. J Biol Chem. 2025. PMID: 40204091 Free PMC article.
-
Expression of a functional Drosophila melanogaster CMP-sialic acid synthetase. Differential localization of the Drosophila and human enzymes.J Biol Chem. 2006 Jun 9;281(23):15929-40. doi: 10.1074/jbc.M512186200. Epub 2006 Mar 14. J Biol Chem. 2006. PMID: 16537535
-
The role of Drosophila cytidine monophosphate-sialic acid synthetase in the nervous system.J Neurosci. 2013 Jul 24;33(30):12306-15. doi: 10.1523/JNEUROSCI.5220-12.2013. J Neurosci. 2013. PMID: 23884937 Free PMC article.
-
CMP-Sialic Acid Synthetase: The Point of Constriction in the Sialylation Pathway.Top Curr Chem. 2015;366:139-67. doi: 10.1007/128_2013_477. Top Curr Chem. 2015. PMID: 24141690 Review.
-
CMP-sialic acid synthetase of the nucleus.Biochim Biophys Acta. 2004 Jul 6;1673(1-2):56-65. doi: 10.1016/j.bbagen.2004.04.006. Biochim Biophys Acta. 2004. PMID: 15238249 Review.
Cited by
-
Sulfated and sialylated N-glycans in the echinoderm Holothuria atra reflect its marine habitat and phylogeny.J Biol Chem. 2020 Mar 6;295(10):3159-3172. doi: 10.1074/jbc.RA119.011701. Epub 2020 Jan 22. J Biol Chem. 2020. PMID: 31969392 Free PMC article.
-
Sialic acids as attachment factors in mosquitoes mediating Japanese encephalitis virus infection.J Virol. 2024 May 14;98(5):e0195923. doi: 10.1128/jvi.01959-23. Epub 2024 Apr 18. J Virol. 2024. PMID: 38634598 Free PMC article.
-
Structural and functional characterization of CMP-N-acetylneuraminate synthetase from Vibrio cholerae.Acta Crystallogr D Struct Biol. 2019 Jun 1;75(Pt 6):564-577. doi: 10.1107/S2059798319006831. Epub 2019 May 31. Acta Crystallogr D Struct Biol. 2019. PMID: 31205019 Free PMC article.
-
CMP-sialic acid synthetase in Drosophila requires N-glycosylation of a noncanonical site.J Biol Chem. 2025 Jun;301(6):108483. doi: 10.1016/j.jbc.2025.108483. Epub 2025 Apr 7. J Biol Chem. 2025. PMID: 40204091 Free PMC article.
-
The generation of 5-N-glycolylneuraminic acid as a consequence of high levels of reactive oxygen species.Glycoconj J. 2023 Aug;40(4):435-448. doi: 10.1007/s10719-023-10121-y. Epub 2023 Jun 2. Glycoconj J. 2023. PMID: 37266899
References
-
- Varki A, Schauer R. Sialic Acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. pp. 199–219. - PubMed
-
- Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. - PubMed
-
- Sellmeier M, Weinhold B, Münster-Kühnel A. CMP-Sialic Acid Synthetase: The Point of Constriction in the Sialylation Pathway. Springer; Berlin Heidelberg: 2013. pp. 1–29. - PubMed
-
- Freeze HH, Elbein AD. Glycosylation Precursors. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. pp. 47–61.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

