Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children's Oncology Group Study AALL0232
- PMID: 27114587
- PMCID: PMC4981974
- DOI: 10.1200/JCO.2015.62.4544
Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children's Oncology Group Study AALL0232
Abstract
Purpose: Survival for children and young adults with high-risk B-acute lymphoblastic leukemia has improved significantly, but 20% to 25% of patients are not cured. Children's Oncology Group study AALL0232 tested two interventions to improve survival.
Patients and methods: Between January 2004 and January 2011, AALL0232 enrolled 3,154 participants 1 to 30 years old with newly diagnosed high-risk B-acute lymphoblastic leukemia. By using a 2 × 2 factorial design, 2,914 participants were randomly assigned to receive dexamethasone (14 days) versus prednisone (28 days) during induction and high-dose methotrexate versus Capizzi escalating-dose methotrexate plus pegaspargase during interim maintenance 1.
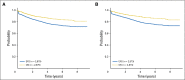
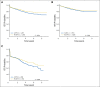
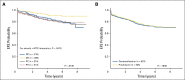
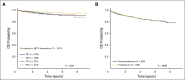
Results: Planned interim monitoring showed the superiority of the high-dose methotrexate regimens, which exceeded the predefined boundary and led to cessation of enrollment in January 2011. At that time, participants randomly assigned to high-dose methotrexate during interim maintenance 1 versus those randomly assigned to Capizzi methotrexate had a 5-year event-free survival (EFS) of 82% versus 75.4% (P = .006). Mature final data showed 5-year EFS rates of 79.6% for high-dose methotrexate and 75.2% for Capizzi methotrexate (P = .008). High-dose methotrexate decreased both marrow and CNS recurrences. Patients 1 to 9 years old who received dexamethasone and high-dose methotrexate had a superior outcome compared with those who received the other three regimens (5-year EFS, 91.2% v 83.2%, 80.8%, and 82.1%; P = .015). Older participants derived no benefit from dexamethasone during induction and experienced excess rates of osteonecrosis.
Conclusion: High-dose methotrexate is superior to Capizzi methotrexate for the treatment of high-risk B-acute lymphoblastic leukemia, with no increase in acute toxicity. Dexamethasone given during induction benefited younger children but provided no benefit and was associated with a higher risk of osteonecrosis among participants 10 years and older.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



References
-
- Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. - PubMed
-
- Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

