Structure-function relationships of peptides forming the calcin family of ryanodine receptor ligands
- PMID: 27114612
- PMCID: PMC4845687
- DOI: 10.1085/jgp.201511499
Structure-function relationships of peptides forming the calcin family of ryanodine receptor ligands
Abstract
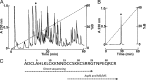
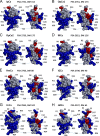
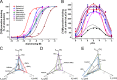
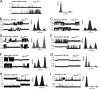
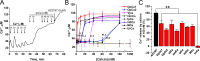
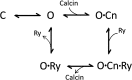
Calcins are a novel family of scorpion peptides that bind with high affinity to ryanodine receptors (RyRs) and increase their activity by inducing subconductance states. Here, we provide a comprehensive analysis of the structure-function relationships of the eight calcins known to date, based on their primary sequence, three-dimensional modeling, and functional effects on skeletal RyRs (RyR1). Primary sequence alignment and evolutionary analysis show high similarity among all calcins (≥78.8% identity). Other common characteristics include an inhibitor cysteine knot (ICK) motif stabilized by three pairs of disulfide bridges and a dipole moment (DM) formed by positively charged residues clustering on one side of the molecule and neutral and negatively charged residues segregating on the opposite side. [(3)H]Ryanodine binding assays, used as an index of the open probability of RyRs, reveal that all eight calcins activate RyR1 dose-dependently with Kd values spanning approximately three orders of magnitude and in the following rank order: opicalcin1 > opicalcin2 > vejocalcin > hemicalcin > imperacalcin > hadrucalcin > maurocalcin >> urocalcin. All calcins significantly augment the bell-shaped [Ca(2+)]-[(3)H]ryanodine binding curve with variable effects on the affinity constants for Ca(2+) activation and inactivation. In single channel recordings, calcins induce the appearance of a subconductance state in RyR1 that has a unique fractional value (∼20% to ∼60% of the full conductance state) but bears no relationship to binding affinity, DM, or capacity to stimulate Ca(2+) release. Except for urocalcin, all calcins at 100 nM concentration stimulate Ca(2+) release and deplete Ca(2+) load from skeletal sarcoplasmic reticulum. The natural variation within the calcin family of peptides offers a diversified set of high-affinity ligands with the capacity to modulate RyRs with high dynamic range and potency.
© 2016 Xiao et al.
Figures


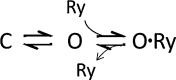

References
-
- Abramson, J.J., Buck E., Salama G., Casida J.E., and Pessah I.N.. 1988. Mechanism of anthraquinone-induced calcium release from skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 263:18750–18758. - PubMed
-
- Altafaj, X., Cheng W., Estève E., Urbani J., Grunwald D., Sabatier J.M., Coronado R., De Waard M., and Ronjat M.. 2005. Maurocalcine and domain A of the II-III loop of the dihydropyridine receptor Cav 1.1 subunit share common binding sites on the skeletal ryanodine receptor. J. Biol. Chem. 280:4013–4016. 10.1074/jbc.C400433200 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

