Exploring the chemical space of influenza neuraminidase inhibitors
- PMID: 27114890
- PMCID: PMC4841240
- DOI: 10.7717/peerj.1958
Exploring the chemical space of influenza neuraminidase inhibitors
Abstract
The fight against the emergence of mutant influenza strains has led to the screening of an increasing number of compounds for inhibitory activity against influenza neuraminidase. This study explores the chemical space of neuraminidase inhibitors (NAIs), which provides an opportunity to obtain further molecular insights regarding the underlying basis of their bioactivity. In particular, a large set of 347 and 175 NAIs against influenza A and B, respectively, was compiled from the literature. Molecular and quantum chemical descriptors were obtained from low-energy conformational structures geometrically optimized at the PM6 level. The bioactivities of NAIs were classified as active or inactive according to their half maximum inhibitory concentration (IC50) value in which IC50 < 1µM and ≥ 10µM were defined as active and inactive compounds, respectively. Interpretable decision rules were derived from a quantitative structure-activity relationship (QSAR) model established using a set of substructure descriptors via decision tree analysis. Univariate analysis, feature importance analysis from decision tree modeling and molecular scaffold analysis were performed on both data sets for discriminating important structural features amongst active and inactive NAIs. Good predictive performance was achieved as deduced from accuracy and Matthews correlation coefficient values in excess of 81% and 0.58, respectively, for both influenza A and B NAIs. Furthermore, molecular docking was employed to investigate the binding modes and their moiety preferences of active NAIs against both influenza A and B neuraminidases. Moreover, novel NAIs with robust binding fitness towards influenza A and B neuraminidase were generated via combinatorial library enumeration and their binding fitness was on par or better than FDA-approved drugs. The results from this study are anticipated to be beneficial for guiding the rational drug design of novel NAIs for treating influenza infections.
Keywords: Chemical space; Combinatorial library enumeration; Data mining; Fragment analysis; Influenza; Molecular docking; Neuraminidase; Neuraminidase inhibitor; QSAR; Scaffold analysis.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
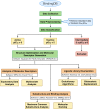
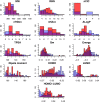








References
-
- Babu YS, Chand P, Bantia S, Kotian P, Dehghani A, El-Kattan Y, Lin TH, Hutchison TL, Elliott AJ, Parker CD, Ananth SL, Horn LL, Laver GW, Montgomery JA. Bcx-1812 (rwj-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. Journal of Medicinal Chemistry. 2000;43(19):3482–3486. doi: 10.1021/jm0002679. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

