Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome
- PMID: 27114901
- PMCID: PMC4833047
- DOI: 10.1016/j.nicl.2016.03.017
Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome
Abstract
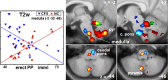
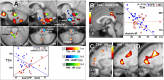
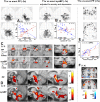
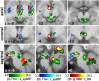
Autonomic changes are often associated with the chronic fatigue syndrome (CFS), but their pathogenetic role is unclear and brain imaging investigations are lacking. The vasomotor centre and, through it, nuclei in the midbrain and hypothalamus play a key role in autonomic nervous system regulation of steady state blood pressure (BP) and heart rate (HR). In this exploratory cross-sectional study, BP and HR, as indicators of autonomic function, were correlated with volumetric and T1- and T2-weighted spin-echo (T1w and T2w) brain MRI in 25 CFS subjects and 25 normal controls (NC). Steady state BP (systolic, diastolic and pulse pressure) and HR in two postures were extracted from 24 h blood pressure monitoring. We performed (1) MRI versus autonomic score interaction-with-group regressions to detect locations where regression slopes differed in the CFS and NC groups (collectively indicating abnormality in CFS), and (2) MRI regressions in the CFS and NC groups alone to detect additional locations with abnormal correlations in CFS. Significant CFS regressions were repeated controlling for anxiety and depression (A&D). Abnormal regressions were detected in nuclei of the brainstem vasomotor centre, midbrain reticular formation and hypothalamus, but also in limbic nuclei involved in stress responses and in prefrontal white matter. Group comparisons of CFS and NC did not find MRI differences in these locations. We propose therefore that these regulatory nuclei are functioning correctly, but that two-way communication between them is impaired in CFS and this affects signalling to/from peripheral effectors/sensors, culminating in inverted or magnified correlations. This single explanation for the diverse abnormal correlations detected here consolidates the conclusion for a brainstem/midbrain nerve conduction deficit inferred earlier (Barnden et al., 2015). Strong correlations were also detected in isolated NC regressions.
Keywords: 1s, 1 sample; 2s, 2 sample; A&D, anxiety and depression; Anxiety and depression; Autonomic; BA, Brodmann Area; BP, blood pressure; Blood pressure; CFS, chronic fatigue syndrome; Cb, cerebellum; Chronic fatigue syndrome; CnF, cuneiform nucleus of the reticular formation; DLPF, dorsolateral prefrontal; FDR, false discovery rate; FWE, family wise error; GM, grey matter; HADS, Hospital Anxiety and Depression Scale; HR, heart rate; Heart rate; Hypothalamus; MRI; Midbrain; NC, normal controls; Nerve conduction; PCC, posterior cingulate cortex; PHg, parahippocampal gyrus; POTS, postural orthostatic tachycardia syndrome; PP, pulse pressure; Posture; RAS, reticular activation system; Regression; SS, symptom score; VBIS, voxel based iterative sensitivity; Vasomotor centre; WM, white matter; ccP, corrected cluster P statistic; diaBP, diastolic blood pressure; sysBP, systolic Blood pressure; uvP, uncorrected voxel P statistic.
Figures
References
-
- Abbott D., Pell G., Pardoe H., Jackson G. Voxel-Based Iterative Sensitivity (VBIS): methods and a validation of intensity scaling for T2-weighted imaging of hippocampal sclerosis. NeuroImage. 2009;44:812–819. - PubMed
-
- Baroncini M., Jissendi P., Balland E., Besson P., Pruvo J., Francke J., Dewailly D., Blond S., Prevot V. MRI atlas of the human hypothalamus. NeuroImage. 2012;59:168–180. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

