Feasible usage of ABO incompatible grafts in living donor liver transplantation
- PMID: 27115002
- PMCID: PMC4824747
- DOI: 10.3978/j.issn.2304-3881.2015.06.02
Feasible usage of ABO incompatible grafts in living donor liver transplantation
Abstract
Background: The use of ABO incompatible (ABOi) graft in living donor liver transplantation (LDLT) has not been an established procedure worldwide.
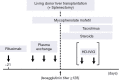
Methods: Four hundred and eight adult LDLTs, using ABOi (n=19) and non-ABOi (n=389) grafts, were performed as a single center experience.
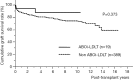
Results: In ABOi-LDLT group (n=19), median isoagglutinin titer before plasma exchange (PE) at LDLT and after LDLT (max) was ×256, ×32 and ×32, respectively. Rituximab was given at 21.8±6.1 days before LDLT and PE was performed 3.7±1.6 times. Although ABOi-LDLTs had increased rate of splenectomy (89.4% vs. 44.7%, P<0.001) and lower portal venous pressure (PVP) at the end of surgery (13.8±1.1 vs. 16.9±0.2 mmHg, P=0.003), other operative factors including graft ischemic time, operative time and blood loss were not different between the groups. Although ABOi-LDLTs had increased incidence of cytomegalovirus infection (52.6% vs. 22.9%, P=0.007), other post-transplant complications including bacterial sepsis and acute rejection were not different between the groups. The 5-year graft survival rate was 87.9% in ABOi-LDLTs and 80.3% in non-ABOi-LDLTs (P=0.373).
Conclusions: ABOi-LDLT could be safely performed, especially under rituximab-based protocol.
Keywords: ABO incompatible (ABOi); living donor liver transplantation (LDLT); rituximab.
Conflict of interest statement
Figures

Similar articles
-
Strategies for ABO Incompatible Liver Transplantation.J Clin Exp Hepatol. 2023 Jul-Aug;13(4):698-706. doi: 10.1016/j.jceh.2022.12.008. Epub 2022 Dec 23. J Clin Exp Hepatol. 2023. PMID: 37440942 Free PMC article. Review.
-
No diffuse intrahepatic biliary stricture after ABO-incompatible adult living donor liver transplantation using tailored rituximab-based desensitization protocol.Ann Transl Med. 2021 Jan;9(1):30. doi: 10.21037/atm-20-4703. Ann Transl Med. 2021. PMID: 33553323 Free PMC article.
-
Comparison of acute kidney injury between ABO-compatible and ABO-incompatible living donor liver transplantation: A propensity matching analysis.Liver Transpl. 2016 Dec;22(12):1656-1665. doi: 10.1002/lt.24634. Liver Transpl. 2016. PMID: 27595780
-
Risk factors for antibody-mediated rejection in ABO blood-type incompatible and donor-specific antibody-positive liver transplantation.Liver Transpl. 2023 Jul 1;29(7):711-723. doi: 10.1097/LVT.0000000000000084. Epub 2023 Feb 8. Liver Transpl. 2023. PMID: 36749821
-
Living donor liver transplantation using ABO-incompatible grafts for chronic and acute liver failure.Surg Today. 2025 Jul 22. doi: 10.1007/s00595-025-03100-3. Online ahead of print. Surg Today. 2025. PMID: 40691743 Review.
Cited by
-
Clinical Relevance of Isoagglutinin Rebound in Adult ABO-Incompatible Living Donor Liver Transplantation.J Pers Med. 2021 Dec 5;11(12):1300. doi: 10.3390/jpm11121300. J Pers Med. 2021. PMID: 34945772 Free PMC article.
-
Feasibility of using marginal liver grafts in living donor liver transplantation.World J Gastroenterol. 2018 Jun 21;24(23):2441-2456. doi: 10.3748/wjg.v24.i23.2441. World J Gastroenterol. 2018. PMID: 29930466 Free PMC article. Review.
-
Strategies for ABO Incompatible Liver Transplantation.J Clin Exp Hepatol. 2023 Jul-Aug;13(4):698-706. doi: 10.1016/j.jceh.2022.12.008. Epub 2022 Dec 23. J Clin Exp Hepatol. 2023. PMID: 37440942 Free PMC article. Review.
-
Outcomes after liver transplantation in accordance with ABO compatibility: A systematic review and meta-analysis.World J Gastroenterol. 2017 Sep 21;23(35):6516-6533. doi: 10.3748/wjg.v23.i35.6516. World J Gastroenterol. 2017. PMID: 29085201 Free PMC article.
-
Computed tomography findings in ABO-incompatible living donor liver transplantation recipients with biliary strictures.Eur Radiol. 2018 Jun;28(6):2572-2581. doi: 10.1007/s00330-017-5226-9. Epub 2018 Jan 2. Eur Radiol. 2018. PMID: 29294154 Clinical Trial.
References
-
- Taketomi A, Kayashima H, Soejima Y, et al. Donor risk in adult-to-adult living donor liver transplantation: impact of left lobe graft. Transplantation 2009;87:445-50. - PubMed
-
- Ikegami T, Shirabe K, Soejima Y, et al. Strategies for successful left-lobe living donor liver transplantation in 250 consecutive adult cases in a single center. J Am Coll Surg 2013;216:353-62. - PubMed
-
- Tanabe M, Shimazu M, Wakabayashi G, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation 2002;73:1959-61. - PubMed
-
- Egawa H, Teramukai S, Haga H, et al. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology 2008;47:143-52. - PubMed
-
- Usui M, Isaji S, Mizuno S, et al. Experiences and problems pre-operative anti-CD20 monoclonal antibody infusion therapy with splenectomy and plasma exchange for ABO-incompatible living-donor liver transplantation. Clin Transplant 2007;21:24-31. - PubMed
LinkOut - more resources
Full Text Sources
