Saline irrigation for chronic rhinosinusitis
- PMID: 27115216
- PMCID: PMC8078614
- DOI: 10.1002/14651858.CD011995.pub2
Saline irrigation for chronic rhinosinusitis
Abstract
Background: This review is one of six looking at the primary medical management options for patients with chronic rhinosinusitis.Chronic rhinosinusitis is common and is characterised by inflammation of the lining of the nose and paranasal sinuses leading to nasal blockage, nasal discharge, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. Nasal saline irrigation is commonly used to improve patient symptoms.
Objectives: To evaluate the effects of saline irrigation in patients with chronic rhinosinusitis.
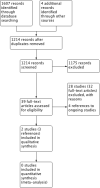
Search methods: The Cochrane ENT Information Specialist searched the ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2015, Issue 9); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 30 October 2015.
Selection criteria: Randomised controlled trials (RCTs) with a follow-up period of at least three months comparing saline delivered to the nose by any means (douche, irrigation, drops, spray or nebuliser) with (a) placebo, (b) no treatment or (c) other pharmacological interventions.
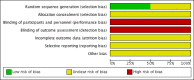
Data collection and analysis: We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease-specific health-related quality of life (HRQL), patient-reported disease severity and the commonest adverse event - epistaxis. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse events of local irritation and discomfort. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
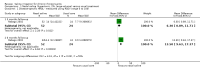
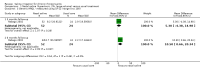
Main results: We included two RCTs (116 adult participants). One compared large-volume (150 ml) hypertonic (2%) saline irrigation with usual treatment over a six-month period; the other compared 5 ml nebulised saline twice a day with intranasal corticosteroids, treating participants for three months and evaluating them on completion of treatment and three months later. Large-volume, hypertonic nasal saline versus usual care One trial included 76 adult participants (52 intervention, 24 control) with or without polyps.Disease-specific HRQL was reported using the Rhinosinusitis Disability Index (RSDI; 0 to 100, 100 = best quality of life). At the end of three months of treatment, patients in the saline group were better than those in the placebo group (mean difference (MD) 6.3 points, 95% confidence interval (CI) 0.89 to 11.71) and at six months there was a greater effect (MD 13.5 points, 95% CI 9.63 to 17.37). We assessed the evidence to be of low quality for the three months follow-up and very low quality for the six months follow-up. Patient-reported disease severity was evaluated using a "single-item sinus symptom severity assessment" but the range of scores is not stated, making it impossible for us to determine the meaning of the data presented.No adverse effects data were collected in the control group but 23% of participants in the saline group experienced side effects including epistaxis. General HRQL was measured using SF-12 (0 to 100, 100 = best quality of life). No difference was found after three months of treatment (low quality evidence) but at six months there was a small difference favouring the saline group, which may not be of clinical significance and has high uncertainty (MD 10.5 points, 95% CI 0.66 to 20.34) (very low quality evidence). Low-volume, nebulised saline versus intranasal corticosteroids One trial included 40 adult participants with polyps. Our primary outcome of disease-specific HRQL was not reported. At the end of treatment (three months) the patients who had intranasal corticosteroids had less severe symptoms (MD -13.50, 95% CI -14.44 to -12.56); this corresponds to a large effect size. We assessed the evidence to be of very low quality.
Authors' conclusions: The two studies were very different in terms of included populations, interventions and comparisons and so it is therefore difficult to draw conclusions for practice. The evidence suggests that there is no benefit of a low-volume (5 ml) nebulised saline spray over intranasal steroids. There is some benefit of daily, large-volume (150 ml) saline irrigation with a hypertonic solution when compared with placebo, but the quality of the evidence is low for three months and very low for six months of treatment.
Conflict of interest statement
Lee Yee Chong: none known.
Karen Head: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Simon Glew: none known.
Glenis Scadding: research grants from GSK and ALK. Honoraria for articles, lectures/chairing, advisory boards and consultancy: Astra Zeneca, Brittania Pharmaceuticals, Capnia, Church & Dwight, Circassia, GSK, Groupo Uriach, Meda, Merck, MSD, Ono Pharmaceuticals, Oxford Therapeutics, Sanofi‐Aventis, Shionogi and UCB. Travel funding from ALK, Bayer, GSK and Meda.
Martin Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
Figures






Update of
References
References to studies included in this review
Cassandro 2015 {published data only}
Rabago 2002 {published data only}
-
- Rabago D, Pasic T, Zgierska A, Mundt M, Barrett B, Maberry R. The efficacy of hypertonic saline nasal irrigation for chronic sinonasal symptoms. Otolaryngology ‐ Head and Neck Surgery 2005;133(1):3‐8. - PubMed
-
- Rabago D, Zgierska A, Mundt M, Barrett B, Bobula J, Maberry R. Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: a randomized controlled trial. Journal of Family Practice 2002;51(12):1049‐55. - PubMed
References to studies excluded from this review
ACTRN12615000154505 {published data only}
-
- ACTRN12615000154505. The effect of xylitol and saline nasal irrigations on the sinus microbiome in patients with chronic rhinosinusitis. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367895 (accessed 17 February 2016).
Bachmann 2000 {published data only}
-
- Bachmann G, Hommel G, Michel O. Effect of irrigation of the nose with isotonic salt solution on adult patients with chronic paranasal sinus disease. European Archives of Oto‐Rhino‐Laryngology 2000;257(10):537‐41. - PubMed
Cho 2010 {published data only}
-
- Cho YS, Kim MS, Chun YH, Kim HH, Kim JT, Lee JS. A prospective randomized open trial of nasal irrigation and nasal decongestant for sinusitis in children. Pediatric Allergy and Respiratory Disease 2010;20(4):232‐7.
Cho 2015 {published data only}
-
- Cho HJ, Min HJ, Chung HJ, Park DY, Seong SY, Yoon JH, et al. Improved outcomes after low‐concentration hypochlorous acid nasal irrigation in pediatric chronic sinusitis. Laryngoscope 2015 Sep 15 [Epub ahead of print]. - PubMed
Cordray 2005 {published data only}
-
- Cordray S, Harjo JB, Miner L. Comparison of intranasal hypertonic dead sea saline spray and intranasal aqueous triamcinolone spray in seasonal allergic rhinitis. Ear, Nose, & Throat Journal 2005;84(7):426‐30. - PubMed
Culig 2010 {published data only}
-
- Culig J, Leppee M, Vceva A, Djanic D. Efficiency of hypertonic and isotonic seawater solutions in chronic rhinosinusitis. Medicinski Glasnik 2010;7(2):116‐23. - PubMed
Desrosiers 2001 {published data only}
-
- Desrosiers MY, Salas‐Prato M. Treatment of chronic rhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large‐particle nebulizer: results of a controlled trial. Otolaryngology ‐ Head and Neck Surgery 2001;125(3):265‐9. - PubMed
Friedman 2006 {published data only}
-
- Friedman M, Vidyasagar R, Joseph N. A randomized, prospective, double‐blind study on the efficacy of dead sea salt nasal irrigations. Laryngoscope 2006;116(6):878‐82. - PubMed
Friedman 2012 {published data only}
-
- Friedman M, Hamilton C, Samuelson CG, Maley A, Wilson MN, Venkatesan TK, et al. Dead Sea salt irrigations vs saline irrigations with nasal steroids for symptomatic treatment of chronic rhinosinusitis: a randomized, prospective double‐blind study. International Forum of Allergy and Rhinology 2012;2(3):252‐7. - PubMed
Garavello 2003 {published data only}
-
- Garavello W, Romagnoli M, Sordo L, Gaini RM, Berardino C, Angrisano A. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: a randomized study. Pediatric Allergy and Immunology 2003;14(2):140‐3. - PubMed
Garavello 2005 {published data only}
-
- Garavello W, Berardino F, Romagnoli M, Sambataro G, Gaini RM. Nasal rinsing with hypertonic solution: an adjunctive treatment for pediatric seasonal allergic rhinoconjunctivitis. International Archives of Allergy and Immunology 2005;137(4):310‐4. - PubMed
Heatley 2000 {published data only}
-
- Heatley D, McConnell KE, Kilie T, Leverson GA. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngology ‐ Head and Neck Surgery 2000;123(2):78‐9. - PubMed
-
- Heatley DG, McConnell KE, Kille TL, Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngology ‐ Head and Neck Surgery 2001;125(1):44‐8. - PubMed
Hunninghake 2012 {published data only}
-
- Hunninghake R, Davis D. A comparison of two nasal irrigation solutions with no treatment in chronic sinusitis sufferers. Townsend Letter 2012;2012(353):66‐73.
Jiang 2014 {published data only}
-
- Jiang RS, Liang KL, Wu SH, Su MC, Chen WK, Lu FJ. Electrolyzed acid water nasal irrigation after functional endoscopic sinus surgery. American Journal of Rhinology & Allergy 2014;28(2):176‐81. - PubMed
NCT00924404 {published data only}
-
- NCT00924404. Randomized controlled trial of xylitol versus saline rinse for chronic sinusitis. clinicaltrials.gov/show/NCT00924404 (accessed 17 February 2016).
NCT01700725 {published data only}
-
- NCT01700725. Nasal irrigation for chronic rhinosinusitis and fatigue in patients with gulf war illness. clinicaltrials.gov/show/NCT01700725 (accessed 17 February 2016).
NCT02097576 {published data only}
-
- NCT02097576. Effectiveness of manuka honey/saline nasal rinses as an adjunct to standard medical therapy for chronic rhinosinusitis: a prospective clinical trial. clinicaltrials.gov/show/NCT02097576 (accessed 17 February 2016).
Ottaviano 2011 {published data only}
-
- Ottaviano G, Marioni G, Staffieri C, Giacomelli L, Marchese‐Ragona R, Bertolin A, et al. Effects of sulfurous, salty, bromic, iodic thermal water nasal irrigations in nonallergic chronic rhinosinusitis: a prospective, randomized, double‐blind, clinical, and cytological study. American Journal of Otolaryngology 2011;32(3):235‐9. - PubMed
Passali 2007 {published data only}
-
- Passali D, Fiorella R, Camaioni A, Villari G, Mora E, Passali GC, et al. Glucan solution nasal spray vs saline in the treatment of chronic rhinosinusitis: a multi‐centric double blind randomised clinical trial. Clinica Terapeutica 2007;158(2):139‐45. - PubMed
Passali 2008 {published data only}
-
- Passali D, Lauriello M, Passali GC, Passali FM, Cassano M, Cassano P, et al. Clinical evaluation of the efficacy of Salsomaggiore (Italy) thermal water in the treatment of rhinosinusal pathologies. Clinica Terapeutica 2008;159(3):181‐8. - PubMed
Passali 2008a {published data only}
-
- Passali FM, Crisanti A, Passali GC, Cianfrone F, Bocchi M, Messineo G, et al. Efficacy of inhalation therapy with water of Salsomaggiore (Italy) in chronic and recurrent nasosinusal inflammation treatment. Clinica Terapeutica 2008;159(3):175‐80. - PubMed
Pynnonen 2007 {published data only}
-
- Pynnonen MA, Mukerji SS, Kim HM, Adams ME, Terrell JE. Nasal saline for chronic sinonasal symptoms: a randomized controlled trial. Archives of Otolaryngology‐‐Head & Neck Surgery 2007;133(11):1115‐20. - PubMed
Rogkakou 2005 {published data only}
-
- Rogkakou A, Guerra L, Massacane P, Baiardini I, Baena‐Cagnani R, Zanella C, et al. Effects on symptoms and quality of life of hypertonic saline nasal spray added to antihistamine in persistent allergic rhinitis ‐ a randomized controlled study. Allergie et Immunologie 2005;37(9):353‐6. - PubMed
Salami 2000 {published data only}
-
- Salami A, Dellepiane M, Strinati F, Guastini L, Mora R. Sulphurous thermal water inhalations in the treatment of chronic rhinosinusitis. Rhinology 2010;48(1):71‐6. - PubMed
Shoseyov 1998 {published data only}
-
- Shoseyov D, Bibi H, Shai P, Shoseyov N, Shazberg G, Hurvitz H. Treatment with hypertonic saline versus normal saline nasal wash of pediatric chronic sinusitis. Journal of Allergy and Clinical Immunology 1998;101(5):602‐5. - PubMed
-
- Slawson D. Is hypertonic saline wash effective in the treatment of pediatric chronic sinusitis?. Evidence‐Based Practice 1998;1(8):6.
Taccariello 1999 {published data only}
-
- Taccariello M, Parikh A, Darby Y, Scadding G. Nasal douching as a valuable adjunct in the management of chronic rhinosinusitis. Rhinology 1999;37(1):29‐32. - PubMed
Ural 2009 {published data only}
-
- Ural A, Oktemer TK, Kizil Y, Ileri F, Uslu S. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: clinical study. Journal of Laryngology & Otology 2009;123(5):517‐21. - PubMed
Wendeler 1997 {published data only}
-
- Wendeler HM, Muller J, Dieler R, Helms J. Nasal irrigation using isotonic Emser salt solution in patients with chronic rhinosinusitis. Oto‐Rhino‐Laryngol Nova 1997;7(5‐6):254‐8.
References to ongoing studies
ISRCTN88204146 {published data only}
-
- ISRCTN88204146. A primary care randomised controlled trial of nasal irrigation and steam inhalation for recurrent sinusitis. www.isrctn.com/ISRCTN88204146 (accessed 17 February 2016).
NCT00335309 {published data only}
-
- NCT00335309. Maxillary sinus irrigation in the management of chronic rhinosinusitis. clinicaltrials.gov/show/NCT00335309 (accessed 3 March 2016).
NCT02582099 {published data only}
-
- NCT02582099. Comparison of the efficacy and complication of gentamicin and normal saline nasal irrigation in chronic rhinosinusitis and recurrent sinusitis: a double blind randomized control trial. clinicaltrials.gov/show/NCT02582099 (accessed 7 February 2016).
TCTR20140323002 {published data only}
-
- TCTR20140323002. The effect of warm saline irrigation on mucociliary function in patients with chronic rhinosinusitis. www.clinicaltrials.in.th (accessed 17 February 2016).
Additional references
Balk 2012
-
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within‐arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. - PubMed
Brown 2004
-
- Brown CL, Graham SM. Nasal irrigations: good or bad?. Current Opinion in Otolaryngology & Head and Neck Surgery 2004;12(1):9‐13. [PUBMED: 14712112] - PubMed
Cho 2012
Chong 2016a
Chong 2016b
Cohen 1998
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988.
DeMarcantonio 2011
-
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. - PubMed
Ebbens 2010
-
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. - PubMed
Ebbens 2011
-
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. - PubMed
Egger 1997
EPOS 2007
-
- Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. - PubMed
EPOS 2012
-
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. - PubMed
Gliklich 1995
-
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Harvey 2007
Hastan 2011
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. - PubMed
Head 2016a
Head 2016b
Head 2016c
Jaeschke 1989
-
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407‐15. [PUBMED: 2691207] - PubMed
Kern 2008
Keswani 2012
Kirtsreesakul 2012
Larsen 2004
-
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. - PubMed
Rabago 2006
Ragab 2004
-
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. - PubMed
Ragab 2010
-
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. - PubMed
Revicki 2008
-
- Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient‐reported outcomes. Journal of Clinical Epidemiology 2008;61(2):102‐9. [PUBMED: 18177782] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Talbot 1997
-
- Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope 1997;107(4):500‐3. - PubMed
Tan 2011
Tomassen 2011
-
- Tomassen P, Zele T, Zhang N, Perez‐ Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. - PubMed
van Drunen 2009
-
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. - PubMed
Vlckova 2009
-
- Vlckova I, Navrátil P, Kana R, Pavlicek P, Chrbolka P, Djupesland PG. Effective treatment of mild‐to‐moderate nasal polyposis with fluticasone delivered by a novel device. Rhinology 2009;47(4):419‐26. - PubMed
Zhang 2008
-
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. - PubMed
Zhang 2009
-
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

