Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis
- PMID: 27115217
- PMCID: PMC9393647
- DOI: 10.1002/14651858.CD011996.pub2
Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis
Abstract
Background: This review is one of six looking at the primary medical management options for patients with chronic rhinosinusitis.Chronic rhinosinusitis is common and is characterised by inflammation of the lining of the nose and paranasal sinuses leading to nasal blockage, rhinorrhoea, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. The use of topical (intranasal) corticosteroids has been widely advocated for the treatment of chronic rhinosinusitis given the belief that inflammation is a major component of this condition.
Objectives: To assess the effects of intranasal corticosteroids in people with chronic rhinosinusitis.
Search methods: The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2015, Issue 8); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 11 August 2015.
Selection criteria: Randomised controlled trials (RCTs) with a follow-up period of at least three months comparing intranasal corticosteroids (e.g. beclomethasone dipropionate, triamcinolone acetonide, flunisolide, budesonide) against placebo or no treatment in patients with chronic rhinosinusitis.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease-specific health-related quality of life (HRQL), patient-reported disease severity and the commonest adverse event - epistaxis. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse events of local irritation or other systemic adverse events. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results: We included 18 RCTs with a total of 2738 participants. Fourteen studies had participants with nasal polyps and four studies had participants without nasal polyps. Only one study was conducted in children. Intranasal corticosteroids versus placebo or no intervention Only one study (20 adult participants without polyps) measured our primary outcome disease-specific HRQL using the Rhinosinusitis Outcome Measures-31 (RSOM-31). They reported no significant difference (numerical data not available) (very low quality evidence).Our second primary outcome, disease severity , was measured using the Chronic Sinusitis Survey in a second study (134 participants without polyps), which found no important difference (mean difference (MD) 2.84, 95% confidence interval (CI) -5.02 to 10.70; scale 0 to 100). Another study (chronic rhinosinusitis with nasal polyps) reported an increased chance of improvement in the intranasal corticosteroids group (RR 2.78, 95% CI 1.76 to 4.40; 109 participants). The quality of the evidence was low.Six studies provided data on at least two of the individual symptoms used in the EPOS 2012 criteria to define chronic rhinosinusitis (nasal blockage, rhinorrhoea, loss of sense of smell and facial pain/pressure). When all four symptoms in the EPOS criteria were available on a scale of 0 to 3 (higher = more severe symptoms), the average MD in change from baseline was -0.26 (95% CI -0.37 to -0.15; 243 participants; two studies; low quality evidence). Although there were more studies and participants when only nasal blockage and rhinorrhoea were considered (MD -0.31, 95% CI -0.38 to -0.24; 1702 participants; six studies), the MD was almost identical to when loss of sense of smell was also considered (1345 participants, four studies; moderate quality evidence).When considering the results for the individual symptoms, benefit was shown in the intranasal corticosteroids group. The effect size was larger for nasal blockage (MD -0.40, 95% CI -0.52 to -0.29; 1702 participants; six studies) than for rhinorrhoea (MD -0.25, 95% CI -0.33 to -0.17; 1702 participants; six studies) or loss of sense of smell (MD -0.19, 95% CI -0.28 to -0.11; 1345 participants; four studies). There was heterogeneity in the analysis for facial pain/pressure (MD -0.27, 95% CI -0.56 to 0.02; 243 participants; two studies). The quality of the evidence was moderate for nasal blockage, rhinorrhoea and loss of sense of smell, but low for facial pain/pressure.There was an increased risk of epistaxis with intranasal corticosteroids (risk ratio (RR) 2.74, 95% CI 1.88 to 4.00; 2508 participants; 13 studies; high quality evidence).Considering our secondary outcome, general HRQL, one study (134 participants without polyps) measured this using the SF-36 and reported a statistically significant benefit only on the general health subscale. The quality of the evidence was very low.It is unclear whether there is a difference in the risk of local irritation (RR 0.94, 95% CI 0.53 to 1.64; 2124 participants; 11 studies) (low quality evidence).None of the studies treated or followed up patients long enough to provide meaningful data on the risk of osteoporosis or stunted growth (children). Other comparisons We identified no other studies that compared intranasal corticosteroids plus co-intervention A versus placebo plus co-intervention A.
Authors' conclusions: Most of the evidence available was from studies in patients with chronic rhinosinusitis with nasal polyps. There is little information about quality of life (very low quality evidence). For disease severity, there seems to be improvement for all symptoms (low quality evidence), a moderate-sized benefit for nasal blockage and a small benefit for rhinorrhoea (moderate quality evidence). The risk of epistaxis is increased (high quality evidence), but these data included all levels of severity; small streaks of blood may not be a major concern for patients. It is unclear whether there is a difference in the risk of local irritation (low quality evidence).
Conflict of interest statement
Lee Yee Chong: none known.
Karen Head: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Figures
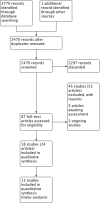

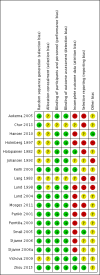










Comment in
-
Cochrane Corner: Extracts from The Cochrane Library.Otolaryngol Head Neck Surg. 2017 Mar;156(3):397-402. doi: 10.1177/0194599816680605. Epub 2016 Dec 20. Otolaryngol Head Neck Surg. 2017. PMID: 28248603
References
References to studies included in this review
Aukema 2005 {published data only}
-
- Aukema AAC, Mulder PGH, Fokkens WJ. Treatment of nasal polyposis and chronic rhinosinusitis with fluticasone propionate nasal drops reduces need for sinus surgery. Journal of Allergy and Clinical Immunology 2005;115(5):1017‐23. - PubMed
-
- Aukema AAC, Mulder PGM, Fokkens WJ. Treatment of nasal polyposis and chronic sinusitis with fluticasone propionate nasal drops (Nasules) reduces symptoms and need for FESS. Proceedings of 19th Congress of the European Rhinologic Society; 2002 Jun 16‐19; Ulm, Germany. 2002.
Chur 2013 {published data only}
-
- Chur V, Small CB, Stryszak P, Teper A. Mometasone furoate nasal spray is safe for the treatment of nasal polyps in pediatric subjects 6‐17 years of age. Journal of Allergy & Clinical Immunology 201;25(2 Suppl 2):AB101.
-
- Chur V, Small CB, Stryszak P, Teper A. Safety of mometasone furoate nasal spray in the treatment of nasal polyps in children. Pediatric Allergy and Immunology 2013;24(1):33‐8. [PUBMED: 23331528] - PubMed
Hansen 2010 {published data only}
-
- Hansen FS, Djupesland PG, Fokkens WJ. Preliminary efficacy of fluticasone delivered by a novel device in recalcitrant chronic rhinosinusitis. Rhinology 2010;48:292‐9. - PubMed
Holmberg 1997 {published data only}
-
- Holmberg K, Juliusson S, Balder B, Smith DL, Richards DH, Karlsson G. Fluticasone propionate aqueous nasal spray in the treatment of nasal polyposis. Annals of Allergy, Asthma, and Immunology 1997;78(3):270‐6. [PUBMED: 9087151] - PubMed
Holopainen 1982 {published data only}
-
- Holopainen E, Grahne B, Malmberg H. Budesonide in the treatment of nasal polyposis. European Journal of Respiratory Diseases 1982;63 Suppl 122:221‐8. - PubMed
Johansen 1993 {published data only}
-
- Johansen LV, Illum P, Kristensen S, Winther L, Vang PS, Synnerstad B. The effect of budesonide (Rhinocort) in the treatment of small and medium‐sized nasal polyps. Clinical Otolaryngology & Allied Sciences 1993;18(6):524‐7. - PubMed
Keith 2000 {published data only}
-
- Holmström M. Clinical performance of fluticasone propionate nasal drops. Allergy: European Journal of Allergy and Clinical Immunology 1999;54(Suppl 53):21‐5. - PubMed
-
- Keith P, Nieminen J, Hollingworth K, Dolovich J. Efficacy and tolerability of fluticasone propionate nasal drops 400 mug daily compared with placebo for the treatment of bilateral polyposis in adults. Clinical and Experimental Allergy 2000;30(10):1460‐8. - PubMed
Lang 1983 {published data only}
-
- Land DA, McNeill J. Double blind controlled study of effect of topical steroids on nasal polyps. Clinical Otolaryngology 1983;8:139.
Lund 1998 {published data only}
-
- Lund VJ, Flood J, Sykes AP, Richards DH. Effect of fluticasone in severe polyposis. Archives of Otolaryngology ‐‐ Head and Neck Surgery 1998;124(5):513‐8. [PUBMED: 9604976] - PubMed
Lund 2004 {published data only}
-
- Lund V, Black S, Laszloy Z, Schrewlius C, Akerlund A. Budesonide aqueous nasal spray (BANS: Rhinocort AquaTM) is effective as monotherapy in stable patients with chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2002;109 Suppl 1:AB886.
-
- Lund VJ, Black JH, Szabo LZ, Schrewelius C, Akerlund A. Efficacy and tolerability of budesonide aqueous nasal spray in chronic rhinosinusitis patients. Rhinology 2004;42(2):57‐62. - PubMed
Mosges 2011 {published data only}
-
- Mosges R, Bachert C, Rudack C, Hauswald B, Klimek L, Spaeth J, et al. Efficacy and safety of mometasone furoate nasal spray in the treatment of chronic rhinosinusitis. Advances in Therapy 2011;28(3):238‐49. - PubMed
Parikh 2001 {published data only}
-
- Parikh A, Scadding GK, Darby Y, Baker RC. Topical corticosteroids in chronic rhinosinusitis: a randomized, double‐blind, placebo‐controlled trial using fluticasone propionate aqueous nasal spray. Rhinology 2001;39(2):75‐9. - PubMed
Penttilla 2000 {published data only}
-
- Holmström M. Clinical performance of fluticasone propionate nasal drops. Allergy: European Journal of Allergy and Clinical Immunology 1999;54(Suppl 53):21‐5. - PubMed
-
- Penttila M, Holstrom M, Poulsen P, Hollingworth K. The efficacy and tolerability of fluticasone propionate (FP) nasal drops 400fig once daily and twice daily compared with placebo in the treatment of nasal polyposis. Allergy 1998;53 Suppl 43:109.
-
- Penttila M, Poulsen P, Hollingworth K, Holmstrom M. Dose‐related efficacy and tolerability of fluticasone propionate nasal drops 400 microg once daily and twice daily in the treatment of bilateral nasal polyposis: a placebo‐controlled randomized study in adult patients. Clinical and Experimental Allergy 2000;30(1):94‐102. [PUBMED: 10606936] - PubMed
Small 2005 {published data only}
-
- Small CB, Hernandez J, Reyes A, Schenkel E, Damiano A, Stryszak P, et al. Efficacy and safety of mometasone furoate nasal spray in nasal polyposis. Journal of Allergy and Clinical Immunology 2005;116(6):1275‐81. [PUBMED: 16337459] - PubMed
Stjarne 2006 {published data only}
-
- Stjarne P, Blomgren K, Caye‐Thomasen P, Salo S, Soderstrom T. The efficacy and safety of once‐daily mometasone furoate nasal spray in nasal polyposis: a randomized, double‐blind, placebo‐controlled study. Acta Oto‐Laryngologica 2006;126(6):606‐12. - PubMed
Stjarne 2006a {published data only}
-
- Stjarne P, Mosges R, Jorissen M, Passali D, Bellussi L, Staudinger H, et al. A randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposis. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2006;132(2):179‐85. - PubMed
Vlckova 2009 {published data only}
-
- Vlckova I, Navrátil P, Kana R, Pavlicek P, Chrbolka P, Djupesland PG. Effective treatment of mild‐to‐moderate nasal polyposis with fluticasone delivered by a novel device. Rhinology 2009;47(4):419‐26. - PubMed
Zhou 2015 {published data only}
-
- A randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the efficacy and safety of 200 mcg bid mometasone furoate nasal spray (MFNS) in the treatment of nasal polyps. www.clinicaltrials.gov/show/NCT01386125 (accessed 10 March 2016).
-
- Zhou B, He G, Liang J, Cheng L, Mehta A, Liu S, et al. Mometasone furoate nasal spray in the treatment of nasal polyposis in Chinese patients: a double‐blind, randomized, placebo‐controlled trial. International Forum of Allergy & Rhinology. 2016;6(1):88‐94. - PubMed
References to studies excluded from this review
ALA 2015 {published data only}
-
- American Lung Association‐Asthma Clinical Research Centers Writing Committee, Dixon AE, Castro M, Cohen RI, Gerald LB, Holbrook JT, et al. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. Journal of Allergy and Clinical Immunology 2015;135(3):701‐9e5. - PMC - PubMed
Albu 2010 {published data only}
-
- Albu S, Gocea A, Mitre I. Preoperative treatment with topical corticoids and bleeding during primary endoscopic sinus surgery. Otolaryngology ‐ Head and Neck Surgery 2010;143(4):573‐8. - PubMed
Bross‐Soriano 2004 {published data only}
-
- Bross‐Soriano D, Arrieta‐Gomez JR, Prado‐Calleros H. Infections after endoscopic polypectomy using nasal steroids. Otolaryngology ‐ Head and Neck Surgery 2004;130(3):319‐22. - PubMed
Cassano 1996 {published data only}
-
- Cassano P, Marini F, Indraccolo AS, Curatoli FP. Corticosteroid therapy in the prevention of recurrent post‐surgical nasal polyposis. Acta Otorhinolaryngologica Italica 1996;16(4):334‐8. - PubMed
Chalton 1985 {published data only}
-
- Chalton R, Mackay IS, Wilson R, Cole P. A double blind, placebo controlled trial of betamethasone nasal drops in the treatment of nasal polyposis. Oto‐Rhino‐Laryngological Research Society (ORS). Abstracts of the meeting held at the Institute of Laryngology and Otology, London October 1985. Clinical Otolaryngology and Allied Sciences 1986;11:389.
Cuenant 1986 {published data only}
-
- Cuenant G, Stipon JP, Plante‐Longchamp G. Efficacy of endonasal neomycin‐tixocortol pivalate irrigation in the treatment of chronic allergic and bacterial sinusitis. ORL: Journal of Oto‐Rhino‐Laryngology 1986;48(4):226‐32. - PubMed
Dijkstra 2004 {published data only}
-
- Dijkstra MD, Ebbens FA, Poublon RML, Fokkens WJ. Fluticasone propionate aqueous nasal spray does not influence the recurrence rate of chronic rhinosinusitis and nasal polyps 1 year after functional endoscopic sinus surgery. Clinical and Experimental Allergy 2004;34(9):1395‐400. - PubMed
-
- Dijkstra MD, Poublon RML, Fokkens WJ. The role of topical steroids after functional endoscopic sinus surgery for chronic sinusitis and/or nasal polyps. Clinical Otolaryngology and Allied Sciences 2001;26(4):341‐2.
Drettner 1982 {published data only}
-
- Drettner B, Ebbesen A, Nilsson M. Prophylactive treatment with flunisolide after polypectomy. Rhinology 1982;20(3):149‐58. - PubMed
Ehnhage 2009 {published data only}
-
- Ehnhage A, Olsson P, Kolbeck KG, Skedinger M, Dahlen B, Alenius M, et al. Functional endoscopic sinus surgery improved asthma symptoms as well as PEFR and olfaction in patients with nasal polyposis. Allergy 2009;64(5):762‐9. - PubMed
-
- Olsson P, Ehnhage A, Nordin S, Stjarne P. Quality of life is improved by endoscopic surgery and fluticasone in nasal polyposis with asthma. Rhinology 2010;48(3):325‐30. - PubMed
el Naggar 1995 {published data only}
-
- Naggar M, Kale S, Aldren C, Martin F. Effect of Beconase nasal spray on olfactory function in post‐nasal polypectomy patients: a prospective controlled trial. Journal of Laryngology and Otology 1995;109(10):941‐4. - PubMed
Filiaci 2000 {published data only}
-
- Filiaci F, Passali D, Puxeddu R, Schrewelius C. A randomized controlled trial showing efficacy of once daily intranasal budesonide in nasal polyposis. Rhinology 2000;38(4):185‐90. - PubMed
Furukido 2005 {published data only}
-
- Furukido K, Takeno S, Ueda T, Yajin K. Cytokine profile in paranasal effusions in patients with chronic sinusitis using the YAMIK sinus catheter with and without betamethasone. European Archives of Oto‐Rhino‐Laryngology 2005;262(1):50‐4. - PubMed
Gulati 2001 {published data only}
Hartwig 1988 {published data only}
-
- Hartwig S, Linden M, Laurent C, Vargo AK, Lindqvist N. Budesonide nasal spray as prophylactic treatment after polypectomy (a double blind clinical trial). Journal of Laryngology and Otology 1988;102(2):148‐51. - PubMed
Jankowski 2001 {published data only}
-
- Jankowski R, Schrewelius C, Bonfils P, Saban Y, Gilain L, Prades JM, et al. Efficacy and tolerability of budesonide aqueous nasal spray treatment in patients with nasal polyps. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2001;127(4):447‐52. - PubMed
Jankowski 2009 {published data only}
-
- Jankowski R, Klossek JM, Attali V, Coste A, Serrano E. Long‐term study of fluticasone propionate aqueous nasal spray in acute and maintenance therapy of nasal polyposis. Allergy 2009;64(6):944‐50. - PubMed
Johansson 2002 {published data only}
-
- Johansson L, Holmberg K, Melen I, Stierna P, Bende M. Sensitivity of a new grading system for studying nasal polyps with the potential to detect early changes in polyp size after treatment with a topical corticosteroid (budesonide). Acta Oto‐Laryngologica 2002;122(1):49‐53. - PubMed
Jorissen 2009 {published data only}
-
- Jorissen M, Bachert C. Effect of corticosteroids on wound healing after endoscopic sinus surgery. Rhinology 2009;47:280‐6. - PubMed
-
- Jorissen M, Bachert C. Effect of postoperative mometasone furoate nasal spray on wound healing following endoscopic sinus surgery. World Allergy Organization Journal. XXth World Allergy Organization Congress & VIIth Asia Pacific Congress of Allergology, Asthma and Clinical Immunology, Bangkok, Thailand. 2007; Vol. S318:Abstract No. 997.
Jurkiewicz 2004 {published data only}
-
- Jurkiewicz D, Zielnik‐Jurkiewicz B, Wojdas A. Effectiveness of fluticasone propionate in nasal polyps treatment. International Review of Allergology and Clinical Immunology 2004;10(1):22‐4.
Kang 2008 {published data only}
-
- Kang IG, Yoon BK, Jung JH, Cha HE, Kim ST. The effect of high‐dose topical corticosteroid therapy on prevention of recurrent nasal polyps after revision endoscopic sinus surgery. American Journal of Rhinology 2008;22(5):497‐501. - PubMed
Kapucu 2012 {published data only}
-
- Kapucu B, Cekin E, Erkul BE, Cincik H, Gungor A, Berber U. The effects of systemic, topical, and intralesional steroid treatments on apoptosis level of nasal polyps. Otolaryngology ‐ Head and Neck Surgery 2012;147(3):563‐7. - PubMed
Karlsson 1982 {published data only}
-
- Karlsson G, Rundcrantz H. A randomized trial in intranasal beclomethasone dipropionate after polypectomy. Rhinology 1982;20(3):144‐8. - PubMed
Keith 1995 {published data only}
-
- Keith PK, Conway M, Evans S, Edney P, Jennings B, Andersson B, et al. A double‐blind comparison of intranasal budesonide dry powder vs placebo in nasal polyposis. Journal of Allergy and Clinical Immunology 1995;95(1):204.
Lavigne 2002 {published data only}
-
- Lavigne F, Cameron L, Renzi PM, Planet JF, Christodoulopoulos P, Lamkioued B, et al. Intrasinus administration of topical budesonide to allergic patients with chronic rhinosinusitis following surgery. Laryngoscope 2002;112(5):858‐64. - PubMed
Lildholdt 1995 {published data only}
-
- Lildholdt T, Rundcrantz H, Bende M, Larsen K. Glucocorticoid treatment for nasal polyps. The use of topical budesonide powder, intramuscular betamethasone, and surgical treatment. Archives of Otolaryngology ‐‐ Head and Neck Surgery 1997;123(6):595‐600. - PubMed
-
- Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polyps: a double‐blind, placebo‐controlled study of budesonide. Clinical Otolaryngology and Allied Sciences 1995;20(1):26‐30. - PubMed
Malmberg 1988 {published data only}
-
- Malmberg H, Holopainen E, Pukander J, Virolainen E, Haye R, Lorentzen P, et al. A one year placebo controlled study of beclomethasone dipropionate (BDP) in the management of nasal polyposis following polypectomy. European Respiratory Journal 1988;1 Suppl 2:202s.
Man 2013 {published data only}
-
- Man LX, Farhood Z, Luong A, Fakhri S, Feldman RM, Orlander PR, et al. The effect of intranasal fluticasone propionate irrigations on salivary cortisol, intraocular pressure, and posterior subcapsular cataracts in postsurgical chronic rhinosinusitis patients. International Forum of Allergy & Rhinology 2013;3(12):953‐7. - PubMed
Mastalerz 1997 {published data only}
-
- Mastalerz L, Milewski M, Duplaga M, Nizankowska E, Szczeklik A. Intranasal fluticasone propionate for chronic eosinophilic rhinitis in patients with aspirin‐induced asthma. Allergy 1997;52(9):895‐900. - PubMed
Meltzer 1993 {published data only}
-
- Meltzer EO, Orgel HA, Backhaus JW, Busse WW, Druce HM, Metzger WJ, et al. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. Journal of Allergy and Clinical Immunology 1993;92(6):812‐23. - PubMed
Mygind 1975 {published data only}
-
- Mygind N. Beclomethasone dipropionate in intranasal treatment [Le dipropionate de beclomethasone en traitement intranasal]. La Nouvelle Presse Médicale 1977;6(15):1319‐21. - PubMed
-
- Mygind N, Pedersen CB, Prytz S, Sorensen H. Treatment of nasal polyps with intranasal beclomethasone dipropionate aerosol. Clinical Allergy 1975;5(2):159‐64. - PubMed
-
- Mygind N, Pedersen CB, Sorensen H. Treatment of nasal polyps. Ugeskrift for Laeger 1976;734(9):548‐52. - PubMed
Optinose 2012 {published data only}
-
- A double blind, randomised, parallel group, placebo‐controlled study to investigate the efficacy and tolerability of intranasal fluticasone propionate delivered by the OptiNose device in adult patients with bilateral nasal polyposis. www.clinicaltrialsregister.eu/ctr‐search/trial/2006‐006340‐60/CZ (accessed 10 March 2016).
Passali 2003 {published data only}
-
- Passali D, Bernstein JM, Passali FM, Damiani V, Passali GC, Bellussi L. Treatment of recurrent chronic hyperplastic sinusitis with nasal polyposis. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2003;129(6):656‐9. - PubMed
Qvarnberg 1992 {published data only}
-
- Qvarnberg Y, Kantola O, Salo J, Toivanen M, Valtonen H, Vuori E. Influence of topical steroid treatment on maxillary sinusitis. Rhinology 1992;30(2):103‐12. - PubMed
Rotenberg 2011 {published data only}
-
- Rotenberg BW, Zhang I, Arra I, Payton KB. Postoperative care for Samter's triad patients undergoing endoscopic sinus surgery: a double‐blinded, randomized controlled trial. Laryngoscope 2011;121:2702–5. - PubMed
Rowe‐Jones 2005 {published data only}
-
- Rowe‐Jones JM, Medcalf M, Durham SR, Richards DH, Mackay IS. Functional endoscopic sinus surgery: 5 year follow up and results of a prospective, randomised, stratified, double‐blind, placebo controlled study of postoperative fluticasone propionate aqueous nasal spray. Rhinology 2005;43(1):2‐10. - PubMed
Ruhno 1990 {published data only}
-
- Ruhno J, Andersson B, Denburg J, Anderson M, Hitch D, Lapp P, et al. A double‐blind comparison of intranasal budesonide with placebo for nasal polyposis. Journal of Allergy and Clinical Immunology 1990;86(6 Pt 1):946‐53. - PubMed
Saunders 1999 {published data only}
-
- Saunders MW, Wheatley AH, George SJ, Lai T, Birchall MA. Do corticosteroids induce apoptosis in nasal polyp inflammatory cells? In vivo and in vitro studies. Laryngoscope 1999;109(5):785‐90. - PubMed
Slifirski 2009 {published data only}
-
- Slifirski J, Rosiek‐Biegus M, Girek M, Fal A. Different effect of topical steroids in nasal polyps' treatment depending on the polyp's cytology. Allergy 2009;64:131.
Stjarne 2009 {published data only}
-
- Stjarne P, Olsson P, Alenius M. Use of mometasone furoate to prevent polyp relapse after endoscopic sinus surgery. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2009;135(3):296‐302. - PubMed
Taub 1968 {published data only}
-
- Taub SJ. A double‐blind labeled study of dexamethasone nasal aerosol vs. placebo in the treatment of nasal polyposis. Journal of Allergy 1968;41(1):10‐3. - PubMed
-
- Taub SJ. Dexamethasone nasal aerosol vs. placebo in the treatment of nasal polyposis. Eye, Ear, Nose & Throat Monthly 1968;47(8):392‐5. - PubMed
Toft 1982 {published data only}
-
- Toft A, Wihl JA, Toxman J, Mygind N. Double‐blind comparison between beclomethasone dipropionate as aerosol and as powder in patients with nasal polyposis. Clinical Allergy 1982;12(4):391‐401. - PubMed
-
- Wihl JA. Topical corticosteroids and nasal reactivity. European Journal of Respiratory Diseases. Supplement 1982;122:205‐10. - PubMed
Tos 1998 {published data only}
-
- Tos M, Svendstrup F, Arndal H, Orntoft S, Jakobsen J, Borum P, et al. Efficacy of an aqueous and a powder formulation of nasal budesonide compared in patients with nasal polyps. American Journal of Rhinology 1998;12(3):183‐9. - PubMed
Vento 2012 {published data only}
-
- Vento SI, Blomgren K, Hytonen M, Simola M, Malmberg H. Prevention of relapses of nasal polyposis with intranasal triamcinolone acetonide after polyp surgery: a prospective double‐blind, placebo‐controlled, randomised study with a 9‐month follow‐up. Clinical Otolaryngology 2012;37(2):117‐23. - PubMed
Virolainen 1980 {published data only}
-
- Virolainen E, Puhakka H. The effect of intranasal beclomethasone dipropionate on the recurrence of nasal polyps after ethmoidectomy. Rhinology 1980;18(1):9‐18. - PubMed
Wang 2015 {published data only}
-
- NCT02024659. Effects and safety of budesonide inhalation suspension via transnasal nebulization in eosinophilic chronic rhinosinusitis with nasal polyps. clinicaltrials.gov/show/NCT02024659 (access 19 February 2016).
-
- Wang C, Lou H, Wang X, Wang Y, Fan E, Li Y, et al. Effect of budesonide transnasal nebulization in patients with eosinophilic chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 2015;135(4):922‐9.e6. - PubMed
References to studies awaiting assessment
Bachert 2004 {published data only}
-
- Bachert C, Bertrand B, Claes J, Clement PAR, Daele J, Demanez J‐P, et al. Efficacy of budesonide powder treatments in nasal polyposis. Proceedings of the 3rd International Consensus Conference on Nasal Polyposis; 2004 Apr 23‐25; Brussels, Belgium. 2004.
Meln 2004 {published data only}
-
- Meln I, Johansson L, Holmberg K, Stierna P, Bende M. Results from treatment with topical steroids. Proceedings of the 3rd International Consensus Conference on Nasal Polyposis; 2004 Apr 23‐25; Brussels, Belgium. 2004:335.
Pisano 2000 {published data only}
-
- Pisano G, Tricarico D, Ascione E, Varricchio AM. Management of nasal polyposis: efficacy of intranasal corticosteroid with hypertonic solution. Proceedings of XVIII Congress of European Rhinologic Society and XIX International Symposium on Infection and Allergy of the Nose; 2000 Jun 25‐29; Barcelona, Spain. 2000:AB414.
Riem 2005 {published data only}
-
- Riem L. Mometasone fuorate for the treatment of nasal polyps. Medizinische Welt 2005;56:224.
Ygind 1996 {published data only}
-
- Ygind N, Lindholdt T. Nasal polyps treatment: medical management. Allergy and Asthma Proceedings 1996;17(5):275‐82. - PubMed
References to ongoing studies
NCT01013701 {published data only}
-
- Compare the effects of fluticasone furoate nasal spray vs placebo in patients with nasal polypoid disease. https://clinicaltrials.gov/ct2/show/NCT01013701 (accessed 10 March 2016).
NCT01622569 {published data only}
-
- NCT01622569. Study evaluating the efficacy and safety of intranasal administration of 100, 200, and 400 μg of fluticasone propionate twice a day (bid) using a novel bi directional device in subjects with bilateral nasal polyposis followed by an 8‐week open‐label extension phase to assess safety. https://clinicaltrials.gov/show/NCT01622569 (accessed 27 February 2016).
NCT01624662 {published data only}
-
- NCT01624662. Efficacy and safety study of intranasal administration of 100, 200, and 400 μg of fluticasone propionate twice a day (bid) using a novel bi directional device in subjects with bilateral nasal polyposis followed by an 8‐week open‐label extension phase to assess safety. https://clinicaltrials.gov/show/NCT01624662 (accessed 27 February 2016).
Additional references
Balk 2012
-
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within‐arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. - PubMed
Benninger 2003
-
- Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngology ‐ Head and Neck Surgery 2003;129(Suppl 3):S1‐32. - PubMed
Cho 2012
Chong 2016a
Chong 2016b
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988.
DeMarcantonio 2011
-
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. - PubMed
Ebbens 2010
-
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. - PubMed
Ebbens 2011
-
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. - PubMed
Egger 1997
EPOS 2012
-
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. - PubMed
Fokkens 2007
-
- Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. - PubMed
Gliklich 1995
-
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. - PubMed
Grobler 2008
-
- Grobler A, Weitzel EK, Buele A, Jardeleza C, Cheong YC, Field J, et al. Pre‐ and postoperative sinus penetration of nasal irrigation. Laryngoscope 2008;118(11):2078‐81. - PubMed
Hamilos 2000
-
- Hamilos DL. Chronic sinusitis. Journal of Allergy and Clinical Immunology 2000;106:213‐27. - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Harvey 2009
-
- Harvey RJ, Debnath N, Srubiski A, Bleier B, Schlosser RJ. Fluid residuals and drug exposure in nasal irrigation. Otolaryngology ‐ Head and Neck Surgery 2009;141(6):757‐61. - PubMed
Hastan 2011
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. - PubMed
Head 2016a
Head 2016b
Head 2016c
Jaeschke 1989
-
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled clinical trials 1989;10(4):407‐15. [PUBMED: 2691207] - PubMed
Kalish 2012
Kern 2008
Keswani 2012
Larsen 2004
-
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. - PubMed
McNally 1997
-
- McNally PA, White MW, Kaliner MA. Sinusitis in an allergists' office: analysis of 2000 consecutive cases. Allergy and Asthma Proceedings 1997;18:169‐75. - PubMed
Mullol 2009
-
- Mullol J. Corticosteroid treatment in chronic rhinosinusitis: the possibilities and the limits. Immunology and Allergy Clinics of North America 2009;29(4):657‐68. - PubMed
Ragab 2004
-
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. - PubMed
Ragab 2010
-
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. - PubMed
Revicki 2008
-
- Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient‐reported outcomes. Journal of Clinical Epidemiology 2008;61(2):102‐9. [PUBMED: 18177782] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Snidvongs 2011
Spector 1998
-
- Spector SL, Bernstein IL, Li JT, Berger WE, Kaliner MA, Schuller DE, et al. Parameters for the diagnosis and management of sinusitis. Journal of Allergy and Clinical Immunology 1998;102(6 Pt 2):S107‐44. - PubMed
Tan 2011
Thomas 2013
-
- Thomas WW 3rd, Harvey RJ, Rudmik L, Hwang PH, Schlosser RJ. Distribution of topical agents to the paranasal sinuses: an evidence‐based review with recommendations. International Forum of Allergy and Rhinology 2013;3(9):691‐703. - PubMed
Tomassen 2011
-
- Tomassen P, Zele T, Zhang N, Perez‐ Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. - PubMed
van Drunen 2009
-
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. - PubMed
Zhang 2008
-
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. - PubMed
Zhang 2009
-
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

