Determination of the appropriate propofol infusion rate for outpatient upper gastrointestinal endoscopy-a randomized prospective study
- PMID: 27117223
- PMCID: PMC4845313
- DOI: 10.1186/s12876-016-0463-y
Determination of the appropriate propofol infusion rate for outpatient upper gastrointestinal endoscopy-a randomized prospective study
Abstract
Background: Pain and discomfort related to endoscopy sessions can be alleviated by sedation, which minimizes anxiety and allows safe examination. For outpatient endoscopy, reliable short-term sedation without secondary effects is required. This study aimed to assess the effects of intravenous propofol rates on sedation in outpatients undergoing upper gastrointestinal endoscopy.
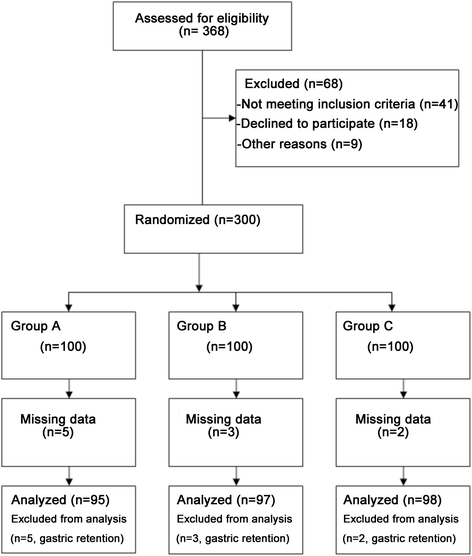
Methods: This randomized prospective study evaluated 300 outpatients submitted to upper gastrointestinal endoscopy. Patients received propofol at 500, 1000 or 2000 ml/h. The primary outcome assessed was hypoxemia incidence. In addition, time to sedation and incidence of hypotension, deep sedation, extremity motor activity, cough, nausea, hiccough, and awareness were evaluated.
Results: Recovery time and incidence of hypoxemia, hypotension, and deep sedation were significantly increased in individuals treated at 2000 ml/h in comparison with values obtained for 500 and 1000 ml/h groups (P < 0.01). Compared with the 500 ml/h group, motor activity of the extremities, cough, nausea, hiccough, and awareness were significantly decreased and the mean scores for endoscopist's and patients' satisfaction were significantly increased in the 1000 and 2000 ml/h groups (P < 0.01).
Conclusion: Propofol infused at 1000 ml/h appeared to be the most suitable infusion rate for outpatient upper gastrointestinal endoscopy.
Registration number: ChiCTR-TRC-14004786 ; Registration date: 2014-06-04.
Keywords: Complications; Outpatient; Propofol; Sedation; Upper gastrointestinal endoscopy.
Similar articles
-
An outcome study comparing intravenous sedation with midazolam/fentanyl (conscious sedation) versus propofol infusion (deep sedation) for aesthetic surgery.Plast Reconstr Surg. 2003 Nov;112(6):1683-9; discussion 1690-1. doi: 10.1097/01.PRS.0000086363.34535.A4. Plast Reconstr Surg. 2003. PMID: 14578803
-
A comparison of propofol vs. dexmedetomidine for sedation, haemodynamic control and satisfaction, during esophagogastroduodenoscopy under conscious sedation.J Clin Pharm Ther. 2015 Aug;40(4):419-25. doi: 10.1111/jcpt.12282. Epub 2015 May 13. J Clin Pharm Ther. 2015. PMID: 25970229 Clinical Trial.
-
Safe and effective sedation in endoscopic submucosal dissection for early gastric cancer: a randomized comparison between propofol continuous infusion and intermittent midazolam injection.J Gastroenterol. 2010 Aug;45(8):831-7. doi: 10.1007/s00535-010-0222-8. Epub 2010 Mar 13. J Gastroenterol. 2010. PMID: 20228999 Clinical Trial.
-
Preparation, premedication and surveillance.Endoscopy. 2003 Feb;35(2):103-11. doi: 10.1055/s-2003-37012. Endoscopy. 2003. PMID: 12561003 Review.
-
Registered nurse-administered propofol sedation for upper endoscopy and colonoscopy: Why? When? How?Rev Gastroenterol Disord. 2003 Spring;3(2):70-80. Rev Gastroenterol Disord. 2003. PMID: 12776004 Review.
Cited by
-
Anesthetic effect of different doses of butorphanol in patients undergoing gastroscopy and colonoscopy.BMC Surg. 2021 May 27;21(1):266. doi: 10.1186/s12893-021-01262-8. BMC Surg. 2021. PMID: 34044830 Free PMC article. Clinical Trial.
-
Effect of butorphanol on visceral pain in patients undergoing gastrointestinal endoscopy: a randomized controlled trial.BMC Anesthesiol. 2023 Mar 28;23(1):93. doi: 10.1186/s12871-023-02053-9. BMC Anesthesiol. 2023. PMID: 36977981 Free PMC article. Clinical Trial.
-
A comparative study on the efficacy and safety of propofol combined with different doses of alfentanil in gastroscopy: a randomized controlled trial.J Anesth. 2023 Apr;37(2):201-209. doi: 10.1007/s00540-022-03145-5. Epub 2022 Dec 8. J Anesth. 2023. PMID: 36482231 Clinical Trial.
-
[Effects of remifentanil on awakening of propofol sedated patients submitted to upper gastrointestinal endoscopy: a randomized clinical trial].Braz J Anesthesiol. 2020 May-Jun;70(3):262-270. doi: 10.1016/j.bjan.2020.03.004. Epub 2020 May 12. Braz J Anesthesiol. 2020. PMID: 32482355 Free PMC article. Clinical Trial.
-
Estimation of median effective effect-site concentration (EC50) during target-controlled infusion of propofol for dilatation and curettage - A prospective observational study.Indian J Anaesth. 2022 Mar;66(3):174-179. doi: 10.4103/ija.ija_547_21. Epub 2022 Mar 24. Indian J Anaesth. 2022. PMID: 35497699 Free PMC article.
References
-
- Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, Ortmann M, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anaesthesiologist administration of propofol for GI endoscopy. Eur J Anaesthesiol. 2010;27:1016–30. doi: 10.1097/EJA.0b013e32834136bf. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical