Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis
- PMID: 27117251
- PMCID: PMC4890572
- DOI: 10.1126/scisignal.aad4949
Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis
Abstract
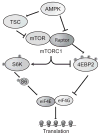
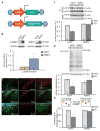
Sleep deprivation is a public health epidemic that causes wide-ranging deleterious consequences, including impaired memory and cognition. Protein synthesis in hippocampal neurons promotes memory and cognition. The kinase complex mammalian target of rapamycin complex 1 (mTORC1) stimulates protein synthesis by phosphorylating and inhibiting the eukaryotic translation initiation factor 4E-binding protein 2 (4EBP2). We investigated the involvement of the mTORC1-4EBP2 axis in the molecular mechanisms mediating the cognitive deficits caused by sleep deprivation in mice. Using an in vivo protein translation assay, we found that loss of sleep impaired protein synthesis in the hippocampus. Five hours of sleep loss attenuated both mTORC1-mediated phosphorylation of 4EBP2 and the interaction between eukaryotic initiation factor 4E (eIF4E) and eIF4G in the hippocampi of sleep-deprived mice. Increasing the abundance of 4EBP2 in hippocampal excitatory neurons before sleep deprivation increased the abundance of phosphorylated 4EBP2, restored the amount of eIF4E-eIF4G interaction and hippocampal protein synthesis to that seen in mice that were not sleep-deprived, and prevented the hippocampus-dependent memory deficits associated with sleep loss. These findings collectively demonstrate that 4EBP2-regulated protein synthesis is a critical mediator of the memory deficits caused by sleep deprivation.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures


Comment in
-
The molecular neurobiology of the sleep-deprived, fuzzy brain.Sci Signal. 2016 Apr 26;9(425):fs7. doi: 10.1126/scisignal.aaf6196. Sci Signal. 2016. PMID: 27117249
Similar articles
-
Dietary polyphenols promote resilience against sleep deprivation-induced cognitive impairment by activating protein translation.FASEB J. 2018 Oct;32(10):5390-5404. doi: 10.1096/fj.201800030R. Epub 2018 Apr 27. FASEB J. 2018. PMID: 29702026 Free PMC article.
-
Transiently increasing cAMP levels selectively in hippocampal excitatory neurons during sleep deprivation prevents memory deficits caused by sleep loss.J Neurosci. 2014 Nov 19;34(47):15715-21. doi: 10.1523/JNEUROSCI.2403-14.2014. J Neurosci. 2014. PMID: 25411499 Free PMC article.
-
Dysregulating mTORC1-4E-BP2 signaling in GABAergic interneurons impairs hippocampus-dependent learning and memory.Learn Mem. 2024 Oct 28;31(10-11):a054018. doi: 10.1101/lm.054018.124. Print 2024 Oct-Nov. Learn Mem. 2024. PMID: 39467641
-
Animal studies on the role of sleep in memory: from behavioral performance to molecular mechanisms.Curr Top Behav Neurosci. 2015;25:183-206. doi: 10.1007/7854_2015_369. Curr Top Behav Neurosci. 2015. PMID: 25680961 Review.
-
Translational Control in the Brain in Health and Disease.Cold Spring Harb Perspect Biol. 2019 Aug 1;11(8):a032912. doi: 10.1101/cshperspect.a032912. Cold Spring Harb Perspect Biol. 2019. PMID: 30082469 Free PMC article. Review.
Cited by
-
Acute Sleep Deprivation Blocks Short- and Long-Term Operant Memory in Aplysia.Sleep. 2016 Dec 1;39(12):2161-2171. doi: 10.5665/sleep.6320. Sleep. 2016. PMID: 27748243 Free PMC article.
-
Single-Nucleus Transcriptome Profiling from the Hippocampus of a PTSD Mouse Model and CBD-Treated Cohorts.Genes (Basel). 2024 Apr 21;15(4):519. doi: 10.3390/genes15040519. Genes (Basel). 2024. PMID: 38674453 Free PMC article.
-
Beyond Emotional and Spatial Processes: Cognitive Dysfunction in a Depressive Phenotype Produced by Long Photoperiod Exposure.PLoS One. 2017 Jan 6;12(1):e0170032. doi: 10.1371/journal.pone.0170032. eCollection 2017. PLoS One. 2017. PMID: 28060930 Free PMC article.
-
The Engram's Dark Horse: How Interneurons Regulate State-Dependent Memory Processing and Plasticity.Front Neural Circuits. 2021 Sep 13;15:750541. doi: 10.3389/fncir.2021.750541. eCollection 2021. Front Neural Circuits. 2021. PMID: 34588960 Free PMC article. Review.
-
Synapses tagged, memories kept: synaptic tagging and capture hypothesis in brain health and disease.Philos Trans R Soc Lond B Biol Sci. 2024 Jul 29;379(1906):20230237. doi: 10.1098/rstb.2023.0237. Epub 2024 Jun 10. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38853570 Free PMC article. Review.
References
-
- Abraham WC, Williams JM. LTP maintenance and its protein synthesis-dependence. Neurobiology of learning and memory. 2008;89:260–268. - PubMed
-
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. - PubMed
-
- Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

