A framework for in vitro systems toxicology assessment of e-liquids
- PMID: 27117495
- PMCID: PMC5309872
- DOI: 10.3109/15376516.2016.1170251
A framework for in vitro systems toxicology assessment of e-liquids
Abstract
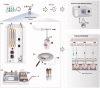
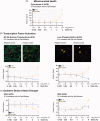
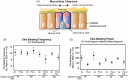
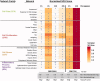
Various electronic nicotine delivery systems (ENDS), of which electronic cigarettes (e-cigs) are the most recognized prototype, have been quickly gaining ground on conventional cigarettes because they are perceived as less harmful. Research assessing the potential effects of ENDS exposure in humans is currently limited and inconclusive. New products are emerging with numerous variations in designs and performance parameters within and across brands. Acknowledging these challenges, we present here a proposed framework for an in vitro systems toxicology assessment of e-liquids and their aerosols, intended to complement the battery of assays for standard toxicity assessments. The proposed framework utilizes high-throughput toxicity assessments of e-liquids and their aerosols, in which the device-to-device variability is minimized, and a systems-level investigation of the cellular mechanisms of toxicity is an integral part. An analytical chemistry investigation is also included as a part of the framework to provide accurate and reliable chemistry data solidifying the toxicological assessment. In its simplest form, the framework comprises of three main layers: (1) high-throughput toxicity screening of e-liquids using primary human cell culture systems; (2) toxicity-related mechanistic assessment of selected e-liquids, and (3) toxicity-related mechanistic assessment of their aerosols using organotypic air-liquid interface airway culture systems. A systems toxicology assessment approach is leveraged to enable in-depth analyses of the toxicity-related cellular mechanisms of e-liquids and their aerosols. We present example use cases to demonstrate the suitability of the framework for a robust in vitro assessment of e-liquids and their aerosols.
Keywords: ENDS; e-Cigarette; high-content screening; organotypic cultures transcriptomics.
Figures













Similar articles
-
Application of a multi-layer systems toxicology framework for in vitro assessment of the biological effects of Classic Tobacco e-liquid and its corresponding aerosol using an e-cigarette device with MESH™ technology.Arch Toxicol. 2019 Nov;93(11):3229-3247. doi: 10.1007/s00204-019-02565-9. Epub 2019 Sep 7. Arch Toxicol. 2019. PMID: 31494692
-
Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung.PLoS One. 2015 Feb 6;10(2):e0116732. doi: 10.1371/journal.pone.0116732. eCollection 2015. PLoS One. 2015. PMID: 25658421 Free PMC article.
-
Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols.Int J Environ Res Public Health. 2014 Oct 30;11(11):11325-47. doi: 10.3390/ijerph111111325. Int J Environ Res Public Health. 2014. PMID: 25361047 Free PMC article.
-
Recent advances in the analysis of electronic cigarette liquids and aerosols: Sample preparation and chromatographic characterization.J Chromatogr A. 2023 Dec 6;1712:464495. doi: 10.1016/j.chroma.2023.464495. Epub 2023 Nov 7. J Chromatogr A. 2023. PMID: 37952386 Review.
-
Critical research gaps in electronic cigarette devices and nicotine aerosols.Int J Pharm. 2021 Jan 25;593:120144. doi: 10.1016/j.ijpharm.2020.120144. Epub 2020 Dec 5. Int J Pharm. 2021. PMID: 33285247 Review.
Cited by
-
E-cigarettes: A novel therapy or a looming catastrophe.Ann Thorac Med. 2021 Jan-Mar;16(1):73-80. doi: 10.4103/atm.ATM_190_20. Epub 2021 Jan 14. Ann Thorac Med. 2021. PMID: 33680128 Free PMC article. Review.
-
Comparative toxicological assessment of cigarettes and new category products via an in vitro multiplex proteomics platform.Toxicol Rep. 2024 May 6;12:492-501. doi: 10.1016/j.toxrep.2024.04.006. eCollection 2024 Jun. Toxicol Rep. 2024. PMID: 38774478 Free PMC article.
-
The flavoring and not the nicotine content is a decisive factor for the effects of refill liquids of electronic cigarette on the redox status of endothelial cells.Toxicol Rep. 2020 Sep 1;7:1095-1102. doi: 10.1016/j.toxrep.2020.08.029. eCollection 2020. Toxicol Rep. 2020. PMID: 32953462 Free PMC article.
-
In Vitro Toxicological Investigation and Risk Assessment of E-Cigarette Aerosols Based on a Novel Solvent-Free Extraction Method.ACS Omega. 2022 Dec 6;7(51):48403-48415. doi: 10.1021/acsomega.2c06663. eCollection 2022 Dec 27. ACS Omega. 2022. PMID: 36591148 Free PMC article.
-
Considerations on dosimetry for in vitro assessment of e-cigarette toxicity.Respir Res. 2022 Dec 17;23(1):358. doi: 10.1186/s12931-022-02286-1. Respir Res. 2022. PMID: 36528600 Free PMC article. Review.
References
-
- Albino AP, Huang X, Jorgensen E, et al. Induction of H2AX phosphorylation in pulmonary cells by tobacco smoke: a new assay for carcinogens. Cell Cycle. 2004;3:1062–8. - PubMed
-
- Balls M. ATLA (Alternatives to Laboratory Animals): past, present and future. Altern Lab Anim. 2010;38:437–41. - PubMed
-
- Berg N, De Wever B, Fuchs HW, et al. . Toxicology in the 21st century-working our way towards a visionary reality. Toxicol In Vitro. 2011;25:874–81. - PubMed
-
- Bijanzadeh M, Ramachandra NB, Mahesh PA, et al. Soluble intercellular adhesion molecule-1 and E-selectin in patients with asthma exacerbation. Lung. 2009;187:315–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
