Bacterial superglue enables easy development of efficient virus-like particle based vaccines
- PMID: 27117585
- PMCID: PMC4847360
- DOI: 10.1186/s12951-016-0181-1
Bacterial superglue enables easy development of efficient virus-like particle based vaccines
Abstract
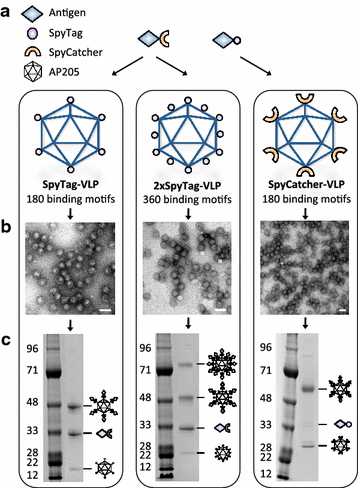
Background: Virus-like particles (VLPs) represent a significant advance in the development of subunit vaccines, combining high safety and efficacy. Their particulate nature and dense repetitive subunit organization makes them ideal scaffolds for display of vaccine antigens. Traditional approaches for VLP-based antigen display require labor-intensive trial-and-error optimization, and often fail to generate dense antigen display. Here we utilize the split-intein (SpyTag/SpyCatcher) conjugation system to generate stable isopeptide bound antigen-VLP complexes by simply mixing of the antigen and VLP components.
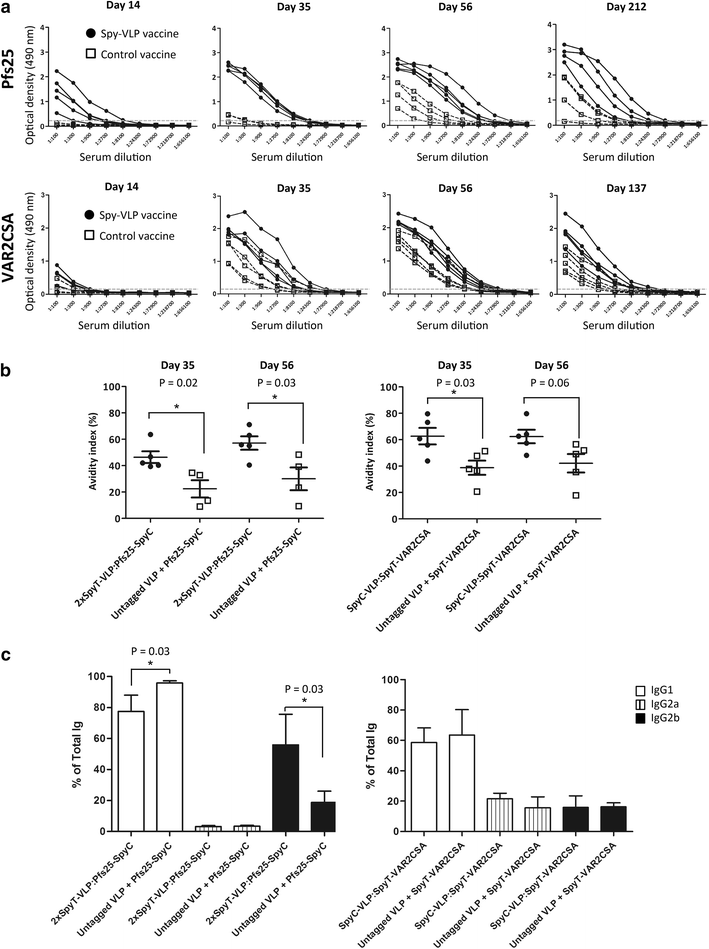
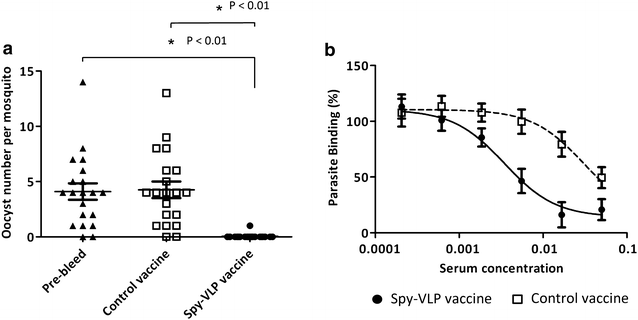
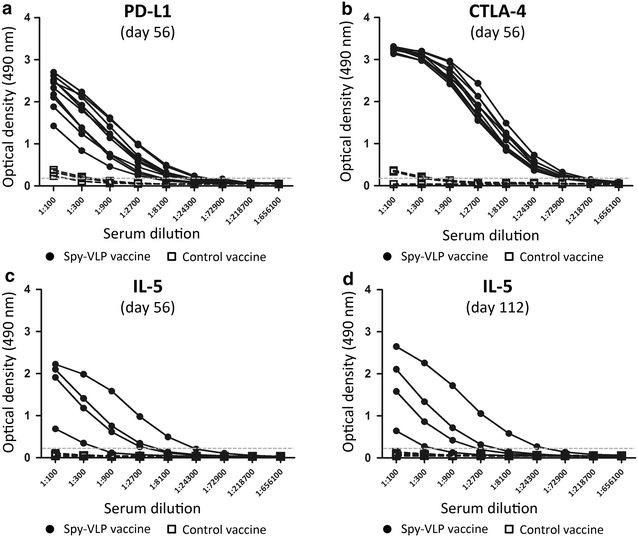
Results: Genetic fusion of SpyTag or SpyCatcher to the N-terminus and/or C-terminus of the Acinetobacter phage AP205 capsid protein resulted in formation of stable, nonaggregated VLPs expressing one SpyCatcher, one SpyTag or two SpyTags per capsid protein. Mixing of spy-VLPs with eleven different vaccine antigens fused to SpyCatcher or SpyTag resulted in formation of antigen-VLP complexes with coupling efficiencies (% occupancy of total VLP binding sites) ranging from 22-88 %. In mice, spy-VLP vaccines presenting the malaria proteins Pfs25 or VAR2CSA markedly increased antibody titer, affinity, longevity and functional efficacy compared to corresponding vaccines employing monomeric proteins. The spy-VLP vaccines also effectively broke B cell self-tolerance and induced potent and durable antibody responses upon vaccination with cancer or allergy-associated self-antigens (PD-L1, CTLA-4 and IL-5).
Conclusions: The spy-VLP system constitutes a versatile and rapid method to develop highly immunogenic VLP-based vaccines. Our data provide proof-of-concept for the technology's ability to present complex vaccine antigens to the immune system and elicit robust functional antibody responses as well as to efficiently break B cell self-tolerance. The spy-VLP-system may serve as a generic tool for the cost-effective development of effective VLP-vaccines against both infectious- and non-communicable diseases and could facilitate rapid and unbiased screening of vaccine candidate antigens.
Figures
Similar articles
-
Bacterial superglue generates a full-length circumsporozoite protein virus-like particle vaccine capable of inducing high and durable antibody responses.Malar J. 2016 Nov 8;15(1):545. doi: 10.1186/s12936-016-1574-1. Malar J. 2016. PMID: 27825348 Free PMC article.
-
Improving the malaria transmission-blocking activity of a Plasmodium falciparum 48/45 based vaccine antigen by SpyTag/SpyCatcher mediated virus-like display.Vaccine. 2017 Jun 27;35(30):3726-3732. doi: 10.1016/j.vaccine.2017.05.054. Epub 2017 May 31. Vaccine. 2017. PMID: 28578824
-
SpyTag/SpyCatcher display of influenza M2e peptide on norovirus-like particle provides stronger immunization than direct genetic fusion.Front Cell Infect Microbiol. 2023 Jun 22;13:1216364. doi: 10.3389/fcimb.2023.1216364. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37424789 Free PMC article.
-
Affinity selection of epitope-based vaccines using a bacteriophage virus-like particle platform.Curr Opin Virol. 2015 Apr;11:76-82. doi: 10.1016/j.coviro.2015.03.005. Epub 2015 Mar 29. Curr Opin Virol. 2015. PMID: 25829254 Free PMC article. Review.
-
Current status and future directions of fish vaccines employing virus-like particles.Fish Shellfish Immunol. 2020 May;100:49-57. doi: 10.1016/j.fsi.2020.02.060. Epub 2020 Mar 1. Fish Shellfish Immunol. 2020. PMID: 32130976 Review.
Cited by
-
Development of SpyTag/SpyCatcher-Bacmid Expression Vector System (SpyBEVS) for Protein Bioconjugations Inside of Silkworms.Int J Mol Sci. 2019 Aug 29;20(17):4228. doi: 10.3390/ijms20174228. Int J Mol Sci. 2019. PMID: 31470538 Free PMC article.
-
A cVLP-Based Vaccine Displaying Full-Length PCSK9 Elicits a Higher Reduction in Plasma PCSK9 Than Similar Peptide-Based cVLP Vaccines.Vaccines (Basel). 2022 Dec 20;11(1):2. doi: 10.3390/vaccines11010002. Vaccines (Basel). 2022. PMID: 36679847 Free PMC article.
-
Synthetic biology for bioengineering virus-like particle vaccines.Biotechnol Bioeng. 2019 Apr;116(4):919-935. doi: 10.1002/bit.26890. Epub 2018 Dec 31. Biotechnol Bioeng. 2019. PMID: 30597533 Free PMC article. Review.
-
A VAR2CSA:CSP conjugate capable of inducing dual specificity antibody responses.Afr Health Sci. 2017 Jun;17(2):373-381. doi: 10.4314/ahs.v17i2.11. Afr Health Sci. 2017. PMID: 29062332 Free PMC article.
-
Construct design, production, and characterization of Plasmodium falciparum 48/45 R0.6C subunit protein produced in Lactococcus lactis as candidate vaccine.Microb Cell Fact. 2017 May 31;16(1):97. doi: 10.1186/s12934-017-0710-0. Microb Cell Fact. 2017. PMID: 28569168 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

