Tumor-Derived Exosomes and Their Role in Cancer Progression
- PMID: 27117662
- PMCID: PMC5382933
- DOI: 10.1016/bs.acc.2015.12.005
Tumor-Derived Exosomes and Their Role in Cancer Progression
Abstract
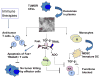
Tumor cells actively produce, release, and utilize exosomes to promote tumor growth. Mechanisms through which tumor-derived exosomes subserve the tumor are under intense investigation. These exosomes are information carriers, conveying molecular and genetic messages from tumor cells to normal or other abnormal cells residing at close or distant sites. Tumor-derived exosomes are found in all body fluids. Upon contact with target cells, they alter phenotypic and functional attributes of recipients, reprogramming them into active contributors to angiogenesis, thrombosis, metastasis, and immunosuppression. Exosomes produced by tumors carry cargos that in part mimic contents of parent cells and are of potential interest as noninvasive biomarkers of cancer. Their role in inhibiting the host antitumor responses and in mediating drug resistance is important for cancer therapy. Tumor-derived exosomes may interfere with cancer immunotherapy, but they also could serve as adjuvants and antigenic components of antitumor vaccines. Their biological roles in cancer development or progression as well as cancer therapy suggest that tumor-derived exosomes are critical components of oncogenic transformation.
Keywords: Cancer progression; Exosome content; Exosomes; Exosomes in immunoregulation; Information transfer; Tumor microenvironment; Tumor-derived exosomes.
© 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The author has no conflict of interest.
Figures
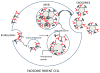

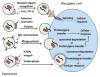

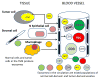

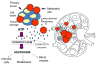

References
-
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. - PubMed
-
- Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, et al. The Simoa HD-1 Analyzer: A Novel Fully Automated Digital Immunoassay Analyzer with Single-Molecule Sensitivity and Multiplexing. J Lab Autom. 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

