The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells
- PMID: 27117702
- PMCID: PMC4860043
- DOI: 10.1016/j.molcel.2016.03.021
The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells
Abstract
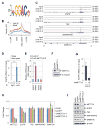
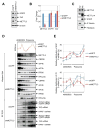
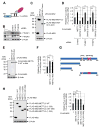
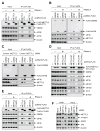
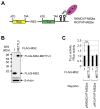
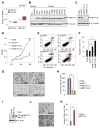
METTL3 is an RNA methyltransferase implicated in mRNA biogenesis, decay, and translation control through N(6)-methyladenosine (m(6)A) modification. Here we find that METTL3 promotes translation of certain mRNAs including epidermal growth factor receptor (EGFR) and the Hippo pathway effector TAZ in human cancer cells. In contrast to current models that invoke m(6)A reader proteins downstream of nuclear METTL3, we find METTL3 associates with ribosomes and promotes translation in the cytoplasm. METTL3 depletion inhibits translation, and both wild-type and catalytically inactive METTL3 promote translation when tethered to a reporter mRNA. Mechanistically, METTL3 enhances mRNA translation through an interaction with the translation initiation machinery. METTL3 expression is elevated in lung adenocarcinoma and using both loss- and gain-of-function studies, we find that METTL3 promotes growth, survival, and invasion of human lung cancer cells. Our results uncover an important role of METTL3 in promoting translation of oncogenes in human lung cancer.
Keywords: EGFR; METTL3; N(6)-methyladenosine; cancer; m(6)A; ribosome; translation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
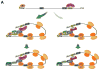
Comment in
-
METTL3 Gains R/W Access to the Epitranscriptome.Mol Cell. 2016 May 5;62(3):323-324. doi: 10.1016/j.molcel.2016.04.024. Mol Cell. 2016. PMID: 27153530
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

