Basal Progenitors Contribute to Repair of the Prostate Epithelium Following Induced Luminal Anoikis
- PMID: 27117783
- PMCID: PMC4939748
- DOI: 10.1016/j.stemcr.2016.03.007
Basal Progenitors Contribute to Repair of the Prostate Epithelium Following Induced Luminal Anoikis
Abstract
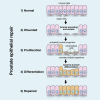
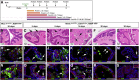
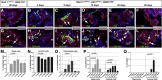
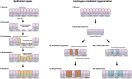
Contact with the extracellular matrix is essential for maintenance of epithelial cells in many tissues, while in its absence epithelial cells can detach and undergo anoikis. Here, we show that anoikis of luminal cells in the prostate epithelium is followed by a program of tissue repair that is mediated in part by differentiation of basal epithelial cells to luminal cells. We describe a mouse model in which inducible deletion of E-cadherin in prostate luminal cells results in their apoptotic cell death by anoikis, in the absence of phenotypic effects in the surrounding stroma. Quantitative assessments of proliferation and cell death in the luminal and basal compartments indicate that basal cells can rapidly generate luminal cells. Thus, our findings identify a role for basal-to-luminal differentiation in prostate epithelial repair, and provide a normal context to analogous processes that may occur during prostate cancer initiation.
Keywords: anoikis; epithelial lineage; prostate; stem cells; tissue repair.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Bonkhoff H., Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98–106. - PubMed
-
- Boussadia O., Kutsch S., Hierholzer A., Delmas V., Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 2002;115:53–62. - PubMed
-
- Chiarugi P., Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008;76:1352–1364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

