Transmission mechanisms of an emerging insect-borne rickettsial pathogen
- PMID: 27117813
- PMCID: PMC4847369
- DOI: 10.1186/s13071-016-1511-8
Transmission mechanisms of an emerging insect-borne rickettsial pathogen
Abstract
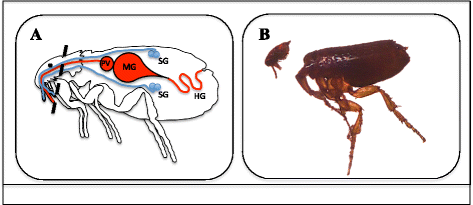
Background: Vector-borne pathogens must overcome arthropod infection and escape barriers (e.g. midgut and salivary glands) during the extrinsic incubation period (EIP) before subsequent transmission to another host. This particular timespan is undetermined for the etiological agent of flea-borne spotted fever (Rickettsia felis). Artificial acquisition of R. felis by blood-feeding cat fleas revealed dissemination to the salivary glands after seven days; however, this length of time is inconsistent with co-feeding studies that produced infectious cat fleas within 24 h of infection. In the current study, we demonstrated that an alternative mechanism is responsible for the early-phase transmission that typifies flea-borne R. felis spread.
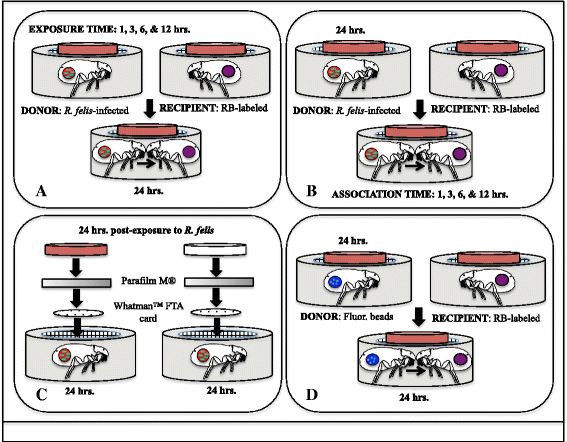
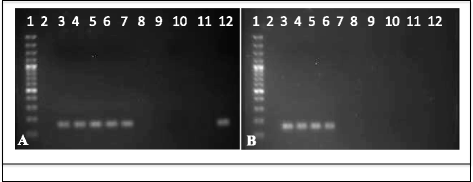
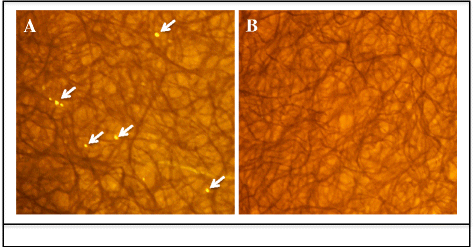
Methods: Co-feeding transmission bioassays were constructed to assess temporal dynamics of R. felis amongst cat fleas, including exposure time to produce infectious fleas and association time to transmit infection to naïve fleas. Additional experiments examined the proportion of R. felis-exposed cat fleas with contaminated mouthparts, as well as the likelihood for cat fleas to release R. felis from their mouthparts following exposure to an infectious bloodmeal. The potential for mechanical transmission of R. felis by co-feeding cat fleas was further examined using fluorescent latex beads, as opposed to a live pathogen, which would not require a biological mechanism to achieve transmission.
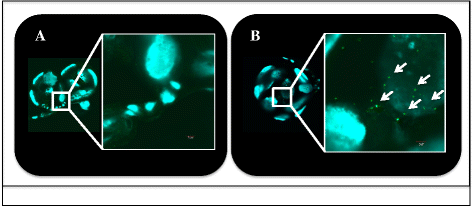
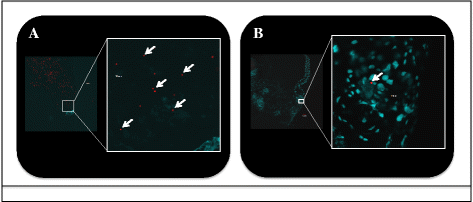
Results: Analyses revealed that R. felis-infected cat fleas were infectious to naïve fleas less than 24 h after exposure to the pathogen, but showed no rickettsial dissemination to the salivary glands during this early-phase transmission. Additionally, the current study revealed that R. felis-infected cat fleas must co-feed with naïve fleas for more than 12 h in order for early-phase transmission to occur. Further evidence supported that contaminated flea mouthparts may be the source of the bacteria transmitted early, and demonstrated that R. felis is released from the mouthparts during brief probing events. Moreover, the use of fluorescent latex beads supports the notion that early-phase transmission of R. felis is a mechanical mechanism.
Conclusions: Determination of the transmission mechanisms utilized by R. felis is essential to fully understand the vulnerability of susceptible vertebrate hosts, including humans, to this pathogen.
Keywords: Cat fleas; Rickettsia felis; Transmission mechanisms.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

