Changing R&D models in research-based pharmaceutical companies
- PMID: 27118048
- PMCID: PMC4847363
- DOI: 10.1186/s12967-016-0838-4
Changing R&D models in research-based pharmaceutical companies
Abstract
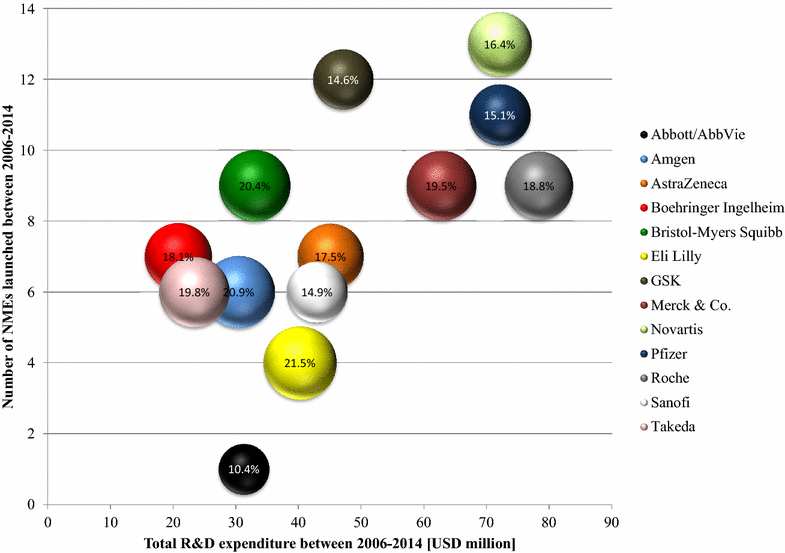
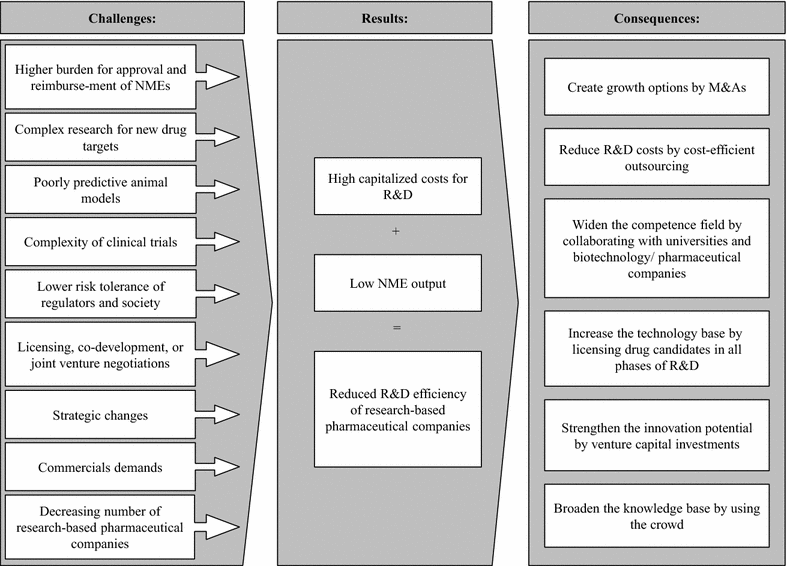
New drugs serving unmet medical needs are one of the key value drivers of research-based pharmaceutical companies. The efficiency of research and development (R&D), defined as the successful approval and launch of new medicines (output) in the rate of the monetary investments required for R&D (input), has declined since decades. We aimed to identify, analyze and describe the factors that impact the R&D efficiency. Based on publicly available information, we reviewed the R&D models of major research-based pharmaceutical companies and analyzed the key challenges and success factors of a sustainable R&D output. We calculated that the R&D efficiencies of major research-based pharmaceutical companies were in the range of USD 3.2-32.3 billion (2006-2014). As these numbers challenge the model of an innovation-driven pharmaceutical industry, we analyzed the concepts that companies are following to increase their R&D efficiencies: (A) Activities to reduce portfolio and project risk, (B) activities to reduce R&D costs, and (C) activities to increase the innovation potential. While category A comprises measures such as portfolio management and licensing, measures grouped in category B are outsourcing and risk-sharing in late-stage development. Companies made diverse steps to increase their innovation potential and open innovation, exemplified by open source, innovation centers, or crowdsourcing, plays a key role in doing so. In conclusion, research-based pharmaceutical companies need to be aware of the key factors, which impact the rate of innovation, R&D cost and probability of success. Depending on their company strategy and their R&D set-up they can opt for one of the following open innovators: knowledge creator, knowledge integrator or knowledge leverager.
Figures
References
-
- Evaluate Pharma. World Preview 2015. 2015. http://info.evaluategroup.com/rs/607-YGS-364/images/wp15.pdf. Accessed 15 Feb 2016.
-
- European Commission—Joint Research Centre. The 2015 EU Industrial R&D investment scoreboard. http://iri.jrc.ec.europa.eu/scoreboard15.html. Accessed 15 Feb 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

