Pharmacokinetic properties of intramuscular versus oral syrup paracetamol in Plasmodium falciparum malaria
- PMID: 27118212
- PMCID: PMC4847232
- DOI: 10.1186/s12936-016-1283-9
Pharmacokinetic properties of intramuscular versus oral syrup paracetamol in Plasmodium falciparum malaria
Abstract
Background: Fever is an inherent symptom of malaria in both adults and children. Paracetamol (acetaminophen) is the recommended antipyretic as it is inexpensive, widely available and has a good safety profile, but patients may not be able to take the oral drug reliably. A comparison between the pharmacokinetics of oral syrup and intramuscular paracetamol given to patients with acute falciparum malaria and high body temperature was performed.
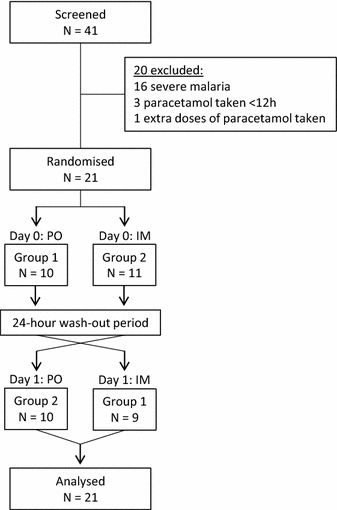
Methods: A randomized, open-label, two-treatment, crossover, pharmacokinetic study of paracetamol dosed orally and intramuscularly was conducted. Twenty-one adult patients with uncomplicated falciparum malaria were randomized to receive a single 600 mg dose of paracetamol either as syrup or intramuscular injection on day 0 followed by a single dose administered by the alternative route on day 1. Paracetamol plasma concentrations were quantified frequently and modelled simultaneously using nonlinear mixed-effects modelling. The final population pharmacokinetic model was used for dose optimization simulations. Relationships between paracetamol concentrations with temperature and parasite half-life were investigated using linear and non-linear regression analyses.
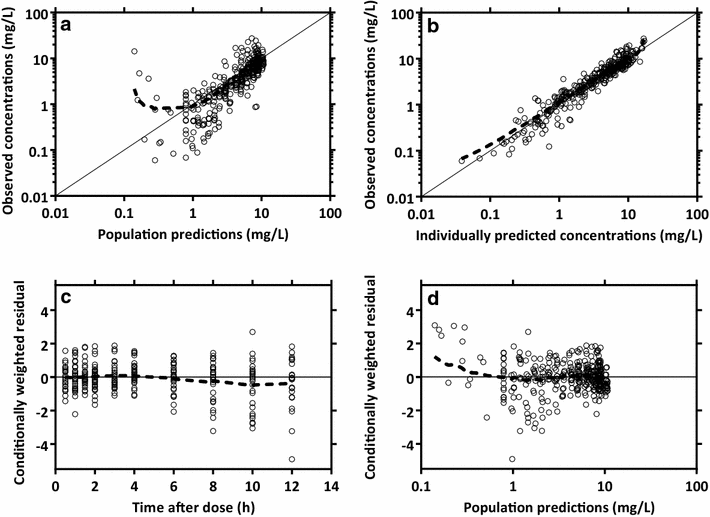
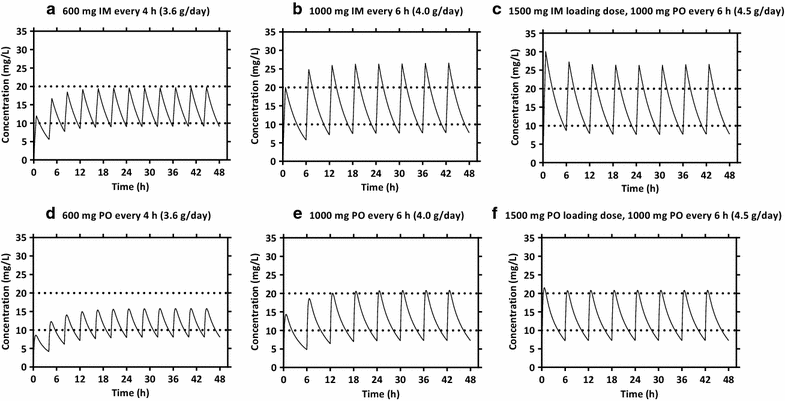
Results: The population pharmacokinetic properties of paracetamol were best described by a two-compartment disposition model, with zero-order and first-order absorption for intramuscular and oral syrup administration, respectively. The relative bioavailability of oral syrup was 84.4 % (95 % CI 68.2-95.1 %) compared to intramuscular administration. Dosing simulations showed that 1000 mg of intramuscular or oral syrup administered six-hourly reached therapeutic steady state concentrations for antipyresis, but more favourable concentration-time profiles were achieved with a loading dose of 1500 mg, followed by a 1000 mg maintenance dose. This ensured that maximum therapeutic concentrations were reached rapidly during the first 6 h. No significant relationships between paracetamol concentrations and temperature or parasite half-life were found.
Conclusions: Paracetamol plasma concentrations after oral syrup and intramuscular administration in patients with acute falciparum malaria were described successfully by a two-compartment disposition model. Relative oral bioavailability compared to intramuscular dosing was estimated as 84.4 % (95 % CI 68.2-95.1 %). Dosing simulations showed that a loading dose followed by six-hourly dosing intervals reduced the time delay to reach therapeutic drug levels after both routes of administration. The safety and efficacy of loading dose paracetamol antipyretic regimens now needs to be established in larger studies.
Keywords: Antipyretic; Falciparum malaria; Intramuscular; NONMEM; Paracetamol; Pharmacokinetics; Randomized crossover trial.
Figures

Similar articles
-
Population pharmacokinetics of orally administered mefloquine in healthy volunteers and patients with uncomplicated Plasmodium falciparum malaria.J Antimicrob Chemother. 2015 Mar;70(3):868-76. doi: 10.1093/jac/dku430. Epub 2014 Nov 4. J Antimicrob Chemother. 2015. PMID: 25377567 Clinical Trial.
-
Safety, tolerability, pharmacokinetics, and antimalarial efficacy of a novel Plasmodium falciparum ATP4 inhibitor SJ733: a first-in-human and induced blood-stage malaria phase 1a/b trial.Lancet Infect Dis. 2020 Aug;20(8):964-975. doi: 10.1016/S1473-3099(19)30611-5. Epub 2020 Apr 8. Lancet Infect Dis. 2020. PMID: 32275867 Clinical Trial.
-
Population pharmacokinetic and pharmacodynamic properties of intramuscular quinine in Tanzanian children with severe Falciparum malaria.Antimicrob Agents Chemother. 2013 Feb;57(2):775-83. doi: 10.1128/AAC.01349-12. Epub 2012 Nov 26. Antimicrob Agents Chemother. 2013. PMID: 23183442 Free PMC article. Clinical Trial.
-
Combined and alternating paracetamol and ibuprofen therapy for febrile children.Evid Based Child Health. 2014 Sep;9(3):675-729. doi: 10.1002/ebch.1978. Evid Based Child Health. 2014. PMID: 25236309 Review.
-
Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration.Malar J. 2011 Sep 13;10:263. doi: 10.1186/1475-2875-10-263. Malar J. 2011. PMID: 21914160 Free PMC article. Review.
Cited by
-
Temperature Dependence of Plasmodium falciparum Erythrocytic Stage Development.Am J Trop Med Hyg. 2019 May;100(5):1191-1195. doi: 10.4269/ajtmh.18-0894. Am J Trop Med Hyg. 2019. PMID: 30938284 Free PMC article.
References
-
- Kitchen SF. Falciparum malaria. In: Boyd MF, editor. Malariology. Philadelphia: WB Saunders; 1949. pp. 966–1045.
-
- World Health Organization. Guidelines for the treatment of malaria. 3rd ed. Switzerland: World Health Organization; 2015. URL: http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf. Accessed 22 Apr 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

