Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS)
- PMID: 27118524
- PMCID: PMC4847011
- DOI: 10.1038/srep24913
Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS)
Abstract
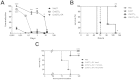
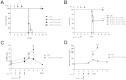
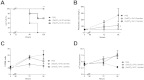
Shiga toxin (Stx)-producing Escherichia coli (STEC) infections are implicated in the development of the life-threatening Hemolytic Uremic Syndrome (HUS). Despite the magnitude of the social and economic problems caused by STEC infections, no licensed vaccine or effective therapy is presently available for human use. Single chain antibodies (VHH) produced by camelids exhibit several advantages in comparison with conventional antibodies, making them promising tools for diagnosis and therapy. In the present work, the properties of a recently developed immunogen, which induces high affinity and protective antibodies against Stx type 2 (Stx2), were exploited to develop VHHs with therapeutic potential against HUS. We identified a family of VHHs against the B subunit of Stx2 (Stx2B) that neutralize Stx2 in vitro at subnanomolar concentrations. One VHH was selected and was engineered into a trivalent molecule (two copies of anti-Stx2B VHH and one anti-seroalbumin VHH). The resulting molecule presented extended in vivo half-life and high therapeutic activity, as demonstrated in three different mouse models of Stx2-toxicity: a single i.v. lethal dose of Stx2, several i.v. incremental doses of Stx2 and intragastrical STEC infection. This simple antitoxin agent should offer new therapeutic options for treating STEC infections to prevent or ameliorate HUS outcome.
Figures



Similar articles
-
Development and Evaluation of a Novel VHH-Based Immunocapture Assay for High-Sensitivity Detection of Shiga Toxin Type 2 (Stx2) in Stool Samples.J Clin Microbiol. 2020 Feb 24;58(3):e01566-19. doi: 10.1128/JCM.01566-19. Print 2020 Feb 24. J Clin Microbiol. 2020. PMID: 31826960 Free PMC article.
-
A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with Shiga toxins 1 and 2.Infect Immun. 2013 Dec;81(12):4592-603. doi: 10.1128/IAI.01033-13. Epub 2013 Sep 30. Infect Immun. 2013. PMID: 24082082 Free PMC article.
-
Protection of mice against Shiga toxin 2 (Stx2)-associated damage by maternal immunization with a Brucella lumazine synthase-Stx2 B subunit chimera.Infect Immun. 2014 Apr;82(4):1491-9. doi: 10.1128/IAI.00027-14. Epub 2014 Jan 13. Infect Immun. 2014. PMID: 24421050 Free PMC article.
-
New Therapeutic Developments against Shiga Toxin-Producing Escherichia coli.Microbiol Spectr. 2014 Oct;2(5). doi: 10.1128/microbiolspec.EHEC-0013-2013. Microbiol Spectr. 2014. PMID: 26104346 Review.
-
Advances in pathogenesis and therapy of hemolytic uremic syndrome caused by Shiga toxin-2.IUBMB Life. 2013 Oct;65(10):827-35. doi: 10.1002/iub.1206. Epub 2013 Sep 6. IUBMB Life. 2013. PMID: 24014500 Review.
Cited by
-
Single Domain Antibody application in bacterial infection diagnosis and neutralization.Front Immunol. 2022 Sep 29;13:1014377. doi: 10.3389/fimmu.2022.1014377. eCollection 2022. Front Immunol. 2022. PMID: 36248787 Free PMC article. Review.
-
Structure-guided design of a potent Clostridiodes difficile toxin A inhibitor.Front Microbiol. 2023 Jan 26;14:1110541. doi: 10.3389/fmicb.2023.1110541. eCollection 2023. Front Microbiol. 2023. PMID: 36778856 Free PMC article.
-
Structural Basis for the Specific Neutralization of Stx2a with a Camelid Single Domain Antibody Fragment.Toxins (Basel). 2018 Mar 1;10(3):108. doi: 10.3390/toxins10030108. Toxins (Basel). 2018. PMID: 29494518 Free PMC article.
-
Therapeutic Strategies to Protect the Central Nervous System against Shiga Toxin from Enterohemorrhagic Escherichia coli.Curr Neuropharmacol. 2021;19(1):24-44. doi: 10.2174/1570159X18666200220143001. Curr Neuropharmacol. 2021. PMID: 32077828 Free PMC article.
-
Nasal immunization with H7 flagellin protects mice against hemolytic uremic syndrome secondary to Escherichia coli O157:H7 gastrointestinal infection.Front Cell Infect Microbiol. 2023 May 16;13:1143918. doi: 10.3389/fcimb.2023.1143918. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37260706 Free PMC article.
References
-
- Kaper J. B., Nataro J. P. & Mobley H. L. Pathogenic Escherichia coli. Nat Rev Microbiol 2(2), 123–140 (2004). - PubMed
-
- Caprioli A., Morabito S., Brugere H. & Oswald E. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet Res 36(3), 289–311 (2005). - PubMed
-
- Rivas M. et al. [The epidemiology of hemolytic uremic syndrome in Argentina. Diagnosis of the etiologic agent, reservoirs and routes of transmission]. Medicina (B Aires) 66 Suppl 3, 27–32 (2006). - PubMed
-
- Noel J. M. & Boedeker E. C. Enterohemorrhagic Escherichia coli: a family of emerging pathogens. Dig Dis 15(1–2), 67–91 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

