Long-acting Neuraminidase Inhibitor Laninamivir Octanoate as Post-exposure Prophylaxis for Influenza
- PMID: 27118785
- PMCID: PMC4946013
- DOI: 10.1093/cid/ciw255
Long-acting Neuraminidase Inhibitor Laninamivir Octanoate as Post-exposure Prophylaxis for Influenza
Abstract
Background: A single administration of laninamivir octanoate, a long-acting neuraminidase inhibitor, has been proven to be effective in the treatment of influenza but not for post-exposure prophylaxis.
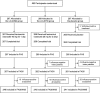
Methods: We conducted a double-blind, multicenter, randomized, placebo-controlled study to determine if a single administration of laninamivir octanoate 40 mg was superior to placebo for post-exposure prophylaxis. Eligible participants who had cohabited with an influenza patient within 48 hours of symptom onset were randomly assigned (1:1:1) to 1 of 3 groups: 40 mg of laninamivir octanoate single administration (LO-40SD), 20 mg of laninamivir octanoate once daily for 2 days (LO-20TD), or placebo. The primary efficacy endpoint was the proportion of participants who developed clinical influenza (defined as influenza virus positive, an axillary temperature >37.5°C, and at least 2 symptoms) over a 10-day period.
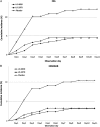
Results: A total of 803 participants were enrolled, with 801 included in the primary analysis. The proportions of participants with clinical influenza were 4.5% (12/267), 4.5% (13/269), and 12.1% (32/265) in the LO-40SD, LO-20TD, and placebo groups, respectively. A single administration of laninamivir octanoate 40 mg significantly reduced the development of influenza compared with placebo (P = .001). The relative risk reductions compared with the placebo group were 62.8% and 63.1% for the LO-40SD and LO-20TD groups, respectively. The incidence of adverse events in the LO-40SD group was similar to that of the LO-20TD and placebo groups.
Conclusions: A single administration of laninamivir octanoate was effective and well tolerated as post-exposure prophylaxis to prevent the development of influenza.
Clinical trials registration: JapicCTI-142679.
Keywords: influenza; laninamivir; neuraminidase inhibitor; post-exposure; prophylaxis.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–24. - PubMed
-
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–75. - PubMed
-
- Watanabe A. A randomized double-blind controlled study of laninamivir compared with oseltamivir for the treatment of influenza in patients with chronic respiratory diseases. J Infect Chemother 2013; 19:89–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

