Human DDX3 protein is a valuable target to develop broad spectrum antiviral agents
- PMID: 27118832
- PMCID: PMC4868442
- DOI: 10.1073/pnas.1522987113
Human DDX3 protein is a valuable target to develop broad spectrum antiviral agents
Abstract
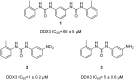
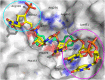
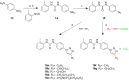
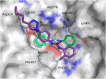
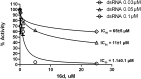
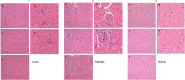
Targeting a host factor essential for the replication of different viruses but not for the cells offers a higher genetic barrier to the development of resistance, may simplify therapy regimens for coinfections, and facilitates management of emerging viral diseases. DEAD-box polypeptide 3 (DDX3) is a human host factor required for the replication of several DNA and RNA viruses, including some of the most challenging human pathogens currently circulating, such as HIV-1, Hepatitis C virus, Dengue virus, and West Nile virus. Herein, we showed for the first time, to our knowledge, that the inhibition of DDX3 by a small molecule could be successfully exploited for the development of a broad spectrum antiviral agent. In addition to the multiple antiviral activities, hit compound 16d retained full activity against drug-resistant HIV-1 strains in the absence of cellular toxicity. Pharmacokinetics and toxicity studies in rats confirmed a good safety profile and bioavailability of 16d. Thus, DDX3 is here validated as a valuable therapeutic target.
Keywords: DDX3; broad spectrum antivirals; coinfections; host factors; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
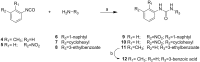

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

