Aerodynamics, sensing and control of insect-scale flapping-wing flight
- PMID: 27118897
- PMCID: PMC4841661
- DOI: 10.1098/rspa.2015.0712
Aerodynamics, sensing and control of insect-scale flapping-wing flight
Erratum in
-
Correction to 'Aerodynamics, sensing and control of insect-scale flapping-wing flight'.Proc Math Phys Eng Sci. 2016 Mar;472(2187):20160096. doi: 10.1098/rspa.2016.0096. Proc Math Phys Eng Sci. 2016. PMID: 31265537 Free PMC article.
Abstract
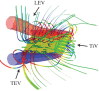
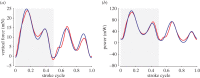
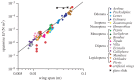
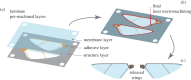
There are nearly a million known species of flying insects and 13 000 species of flying warm-blooded vertebrates, including mammals, birds and bats. While in flight, their wings not only move forward relative to the air, they also flap up and down, plunge and sweep, so that both lift and thrust can be generated and balanced, accommodate uncertain surrounding environment, with superior flight stability and dynamics with highly varied speeds and missions. As the size of a flyer is reduced, the wing-to-body mass ratio tends to decrease as well. Furthermore, these flyers use integrated system consisting of wings to generate aerodynamic forces, muscles to move the wings, and sensing and control systems to guide and manoeuvre. In this article, recent advances in insect-scale flapping-wing aerodynamics, flexible wing structures, unsteady flight environment, sensing, stability and control are reviewed with perspective offered. In particular, the special features of the low Reynolds number flyers associated with small sizes, thin and light structures, slow flight with comparable wind gust speeds, bioinspired fabrication of wing structures, neuron-based sensing and adaptive control are highlighted.
Keywords: biomimicry; flapping flight; insect scale.
Figures




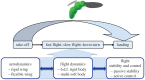
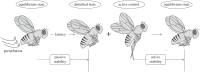
Similar articles
-
Biomechanics and biomimetics in insect-inspired flight systems.Philos Trans R Soc Lond B Biol Sci. 2016 Sep 26;371(1704):20150390. doi: 10.1098/rstb.2015.0390. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27528780 Free PMC article. Review.
-
How oscillating aerodynamic forces explain the timbre of the hummingbird's hum and other animals in flapping flight.Elife. 2021 Mar 16;10:e63107. doi: 10.7554/eLife.63107. Elife. 2021. PMID: 33724182 Free PMC article.
-
Special section on biomimetics of movement.Bioinspir Biomim. 2011 Dec;6(4):040201. doi: 10.1088/1748-3182/6/4/040201. Epub 2011 Nov 29. Bioinspir Biomim. 2011. PMID: 22128305
-
Aerodynamics of a bio-inspired flexible flapping-wing micro air vehicle.Bioinspir Biomim. 2011 Dec;6(4):045002. doi: 10.1088/1748-3182/6/4/045002. Epub 2011 Nov 29. Bioinspir Biomim. 2011. PMID: 22126793
-
Flapping wing aerodynamics: from insects to vertebrates.J Exp Biol. 2016 Apr;219(Pt 7):920-32. doi: 10.1242/jeb.042317. J Exp Biol. 2016. PMID: 27030773 Review.
Cited by
-
Chordwise wing flexibility may passively stabilize hovering insects.J R Soc Interface. 2018 Oct 10;15(147):20180409. doi: 10.1098/rsif.2018.0409. J R Soc Interface. 2018. PMID: 30305421 Free PMC article.
-
Morphology-based classification of the flying capacities of aquatic insects: A first attempt.Curr Zool. 2023 Nov 8;70(5):607-617. doi: 10.1093/cz/zoad047. eCollection 2024 Oct. Curr Zool. 2023. PMID: 39463693 Free PMC article.
-
An advection-deposition-survival model to assess the risk of introduction of vector-borne diseases through the wind: Application to bluetongue outbreaks in Spain.PLoS One. 2018 Mar 22;13(3):e0194573. doi: 10.1371/journal.pone.0194573. eCollection 2018. PLoS One. 2018. PMID: 29566088 Free PMC article.
-
Effects of pterostigma structure on vibrational characteristics during flight of Asian ladybird Harmonia axyridis (Coleoptera: Coccinellidae).Sci Rep. 2020 Jul 9;10(1):11371. doi: 10.1038/s41598-020-68384-6. Sci Rep. 2020. PMID: 32647317 Free PMC article.
-
Flow development and leading edge vorticity in bristled insect wings.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023 Mar;209(2):219-229. doi: 10.1007/s00359-023-01617-x. Epub 2023 Feb 22. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023. PMID: 36810678 Free PMC article.
References
-
- Shyy W, Aono H, Kang C, Liu H. 2013. An introduction to flapping wing aerodynamics. New York, NY: Cambridge University Press.
-
- Dial KP. 1994. Inside look at how birds fly: experimental studies of the inertial and external process controlling flight. In Thirty-Eighths Symp. Proc. Experimental Test Pilots, Lancaster, CA, USA, pp. 301–314. Lancaster, CA: Society of Experimental Test Pilots.
-
- Norberg UM. 1990. Vertebrate flight: mechanics, physiology, morphology, ecology, and evolution. New York, NY: Springer.
-
- Goslow GE, Dial KP, Jenkins FA. 1990. Bird flight: insights and complications. Bioscience 40, 108–115. (doi:10.2307/1311343) - DOI
-
- Shipman P. 1999. Taking wing: archaeopteryx and the evolution of bird flight. New York, NY: Simon and Schuster.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

