Enhanced efficacy with azacytidine and oncolytic BHV-1 in a tolerized cotton rat model of breast adenocarcinoma
- PMID: 27119103
- PMCID: PMC4782958
- DOI: 10.1038/mto.2015.4
Enhanced efficacy with azacytidine and oncolytic BHV-1 in a tolerized cotton rat model of breast adenocarcinoma
Abstract
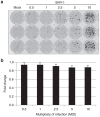
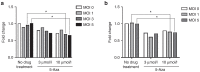
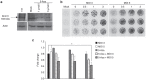
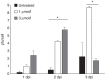
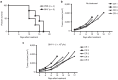
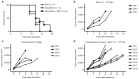
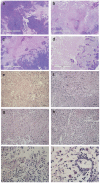
Oncolytic viruses selectively replicate in cancer cells by exploiting biochemical differences between normal and tumor cells. Treatment with epigenetic modifiers such as 5-Azacytidine, a DNA methyltransferase inhibitor, increases the replication and cytotoxicity of oncolytic viruses in vivo and in vitro. The cotton rat is an attractive animal to study oncolytic viruses, as syngeneic models of breast adenocarcinoma and osteosarcoma are well established, and many features of primary and secondary tumor growth recapitulate human disease. Treatment of LCRT breast cancer cells with 5-Azacytidine increases bovine herpesvirus type 1 (BHV-1)-mediated cytotoxicity in vitro, with Chou-Talalay analysis indicating a very strong synergy. In vivo, BHV-1 monotherapy delayed tumor growth but did not improve survival of cotton rats with subcutaneous breast adenocarcinomas. However, combination therapy significantly decreased the incidence of secondary lesions, with enhanced tumor cell clearance and evidence of immune cell infiltration compared to BHV-1 monotherapy. Together, these results warrant further investigation of BHV-1 combination therapy with epigenetic modifiers for the treatment of breast cancer, particularly in the context of the prevention and treatment of secondary lesions.
Figures
Similar articles
-
Oncolytic bovine herpesvirus type 1 as a broad spectrum cancer therapeutic.Curr Opin Virol. 2015 Aug;13:11-6. doi: 10.1016/j.coviro.2015.03.010. Epub 2015 Apr 2. Curr Opin Virol. 2015. PMID: 25846987 Review.
-
Oncolytic bovine herpesvirus type 1 infects and kills breast tumor cells and breast cancer-initiating cells irrespective of tumor subtype.Cancer Gene Ther. 2013 May;20(5):282-9. doi: 10.1038/cgt.2013.18. Epub 2013 Apr 26. Cancer Gene Ther. 2013. PMID: 23618948
-
Handling of the cotton rat in studies for the pre-clinical evaluation of oncolytic viruses.J Vis Exp. 2014 Nov 24;(93):e52232. doi: 10.3791/52232. J Vis Exp. 2014. PMID: 25490047 Free PMC article.
-
Bovine herpesvirus type 1 as a novel oncolytic virus.Cancer Gene Ther. 2010 May;17(5):344-55. doi: 10.1038/cgt.2009.77. Epub 2009 Nov 6. Cancer Gene Ther. 2010. PMID: 19893594
-
Employing tumor hypoxia for oncolytic therapy in breast cancer.J Mammary Gland Biol Neoplasia. 2005 Oct;10(4):311-8. doi: 10.1007/s10911-006-9004-6. J Mammary Gland Biol Neoplasia. 2005. PMID: 16826462 Review.
Cited by
-
The Effects of Oncolytic Pseudorabies Virus Vaccine Strain Inhibited the Growth of Colorectal Cancer HCT-8 Cells In Vitro and In Vivo.Animals (Basel). 2022 Sep 14;12(18):2416. doi: 10.3390/ani12182416. Animals (Basel). 2022. PMID: 36139276 Free PMC article.
-
Induction of Oxidative DNA Damage in Bovine Herpesvirus 1 Infected Bovine Kidney Cells (MDBK Cells) and Human Tumor Cells (A549 Cells and U2OS Cells).Viruses. 2018 Jul 26;10(8):393. doi: 10.3390/v10080393. Viruses. 2018. PMID: 30049996 Free PMC article.
-
Oncolytic BHV-1 Is Sufficient to Induce Immunogenic Cell Death and Synergizes with Low-Dose Chemotherapy to Dampen Immunosuppressive T Regulatory Cells.Cancers (Basel). 2023 Feb 17;15(4):1295. doi: 10.3390/cancers15041295. Cancers (Basel). 2023. PMID: 36831636 Free PMC article.
-
The role of viruses in cancer development versus cancer therapy: An oncological perspective.Cancer Med. 2023 May;12(10):11127-11148. doi: 10.1002/cam4.5694. Epub 2023 Mar 7. Cancer Med. 2023. PMID: 36880311 Free PMC article. Review.
-
DNA Damage Response Differentially Affects BoHV-1 Gene Transcription in Cell Type-Dependent Manners.Biomedicines. 2022 Sep 14;10(9):2282. doi: 10.3390/biomedicines10092282. Biomedicines. 2022. PMID: 36140380 Free PMC article.
References
-
- Cervantes-García, D, Ortiz-López, R, Mayek-Pérez, N and Rojas-Martínez, A (2008). Oncolytic virotherapy. Ann Hepatol 7: 34–45. - PubMed
-
- Kaufman, HL, Kim, DW, DeRaffele, G, Mitcham, J, Coffin, RS and Kim-Schulze, S (2010). Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 17: 718–730. - PubMed
-
- Senzer, NN, Kaufman, HL, Amatruda, T, Nemunaitis, M, Reid, T, Daniels, G et al. (2009). Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27: 5763–5771. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

