ML314: A Biased Neurotensin Receptor Ligand for Methamphetamine Abuse
- PMID: 27119457
- PMCID: PMC4947017
- DOI: 10.1021/acschembio.6b00291
ML314: A Biased Neurotensin Receptor Ligand for Methamphetamine Abuse
Abstract
Pharmacological treatment for methamphetamine addiction will provide important societal benefits. Neurotensin receptor NTR1 and dopamine receptor distributions coincide in brain areas regulating methamphetamine-associated reward, and neurotensin peptides produce behaviors opposing psychostimulants. Therefore, undesirable methamphetamine-associated activities should be treatable with druggable NTR1 agonists, but no such FDA-approved therapeutics exist. We address this limitation with proof-of-concept data for ML314, a small-molecule, brain penetrant, β-arrestin biased, NTR1 agonist. ML314 attenuates amphetamine-like hyperlocomotion in dopamine transporter knockout mice, and in C57BL/6J mice it attenuates methamphetamine-induced hyperlocomotion, potentiates the psychostimulant inhibitory effects of a ghrelin antagonist, and reduces methamphetamine-associated conditioned place preference. In rats, ML314 blocks methamphetamine self-administration. ML314 acts as an allosteric enhancer of endogenous neurotensin, unmasking stoichiometric numbers of hidden NTR1 binding sites in transfected-cell membranes or mouse striatal membranes, while additionally supporting NTR1 endocytosis in cells in the absence of NT peptide. These results indicate ML314 is a viable, preclinical lead for methamphetamine abuse treatment and support an allosteric model of G protein-coupled receptor signaling.
Figures
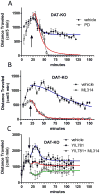



 ) and NT + ML314-1 μM (
) and NT + ML314-1 μM (
 ) fitted curves are shared. Top, bottom, and Log(EC 50) values for each curve were NT (1.01 ±.04, 0.050 ±.045, -9.34±0.13), ML314-1 μM (1.03 ±.04, 0.050 ±.045, -9.64±0.13), and NT ML314-10μM (1.00 ±.07, 0.49 ±.07, -9.48±0.29). Data represent N=4 independent experiments performed in triplicate. E. β-arrestin2-GFP translocation to NTR1 in U2OS cells was determined in the absence and presence of 10-20 μM ML314 that was added simultaneously with NT. Data points represent mean ± sem from N=4 independent experiments in duplicate NT alone)/quadruplicate (NT and ML314) and were fit as in (D) with shared “top”. Top, bottom, and Log(EC 50) values for each curve are: NT (952 ± 37, -111 ± 105, -8.87 ± 0.17) and NT+ ML314 (952 ± 37, 206 ± 69, -8.68 ± 0.18). Data were analyzed and plotted using the software GraphPad Prism version 5.
) fitted curves are shared. Top, bottom, and Log(EC 50) values for each curve were NT (1.01 ±.04, 0.050 ±.045, -9.34±0.13), ML314-1 μM (1.03 ±.04, 0.050 ±.045, -9.64±0.13), and NT ML314-10μM (1.00 ±.07, 0.49 ±.07, -9.48±0.29). Data represent N=4 independent experiments performed in triplicate. E. β-arrestin2-GFP translocation to NTR1 in U2OS cells was determined in the absence and presence of 10-20 μM ML314 that was added simultaneously with NT. Data points represent mean ± sem from N=4 independent experiments in duplicate NT alone)/quadruplicate (NT and ML314) and were fit as in (D) with shared “top”. Top, bottom, and Log(EC 50) values for each curve are: NT (952 ± 37, -111 ± 105, -8.87 ± 0.17) and NT+ ML314 (952 ± 37, 206 ± 69, -8.68 ± 0.18). Data were analyzed and plotted using the software GraphPad Prism version 5.
References
-
- Volkow ND. Methamphetamine, Letter from the Director. 2014 http://www.drugabuse.gov/publications/research-reports/methamphetamine/l....
-
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

