HIPPO-Integrin-linked Kinase Cross-Talk Controls Self-Sustaining Proliferation and Survival in Pulmonary Hypertension
- PMID: 27119551
- PMCID: PMC5074651
- DOI: 10.1164/rccm.201510-2003OC
HIPPO-Integrin-linked Kinase Cross-Talk Controls Self-Sustaining Proliferation and Survival in Pulmonary Hypertension
Abstract
Rationale: Enhanced proliferation and impaired apoptosis of pulmonary arterial vascular smooth muscle cells (PAVSMCs) are key pathophysiologic components of pulmonary vascular remodeling in pulmonary arterial hypertension (PAH).
Objectives: To determine the role and therapeutic relevance of HIPPO signaling in PAVSMC proliferation/apoptosis imbalance in PAH.
Methods: Primary distal PAVSMCs, lung tissue sections from unused donor (control) and idiopathic PAH lungs, and rat and mouse models of SU5416/hypoxia-induced pulmonary hypertension (PH) were used. Immunohistochemical, immunocytochemical, and immunoblot analyses and transfection, infection, DNA synthesis, apoptosis, migration, cell count, and protein activity assays were performed in this study.
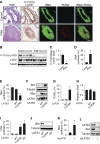
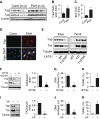
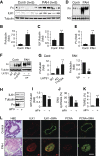
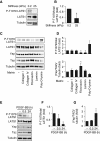
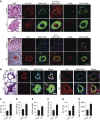
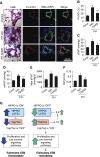
Measurements and main results: Immunohistochemical and immunoblot analyses demonstrated that the HIPPO central component large tumor suppressor 1 (LATS1) is inactivated in small remodeled pulmonary arteries (PAs) and distal PAVSMCs in idiopathic PAH. Molecular- and pharmacology-based analyses revealed that LATS1 inactivation and consequent up-regulation of its reciprocal effector Yes-associated protein (Yap) were required for activation of mammalian target of rapamycin (mTOR)-Akt, accumulation of HIF1α, Notch3 intracellular domain and β-catenin, deficiency of proapoptotic Bim, increased proliferation, and survival of human PAH PAVSMCs. LATS1 inactivation and up-regulation of Yap increased production and secretion of fibronectin that up-regulated integrin-linked kinase 1 (ILK1). ILK1 supported LATS1 inactivation, and its inhibition reactivated LATS1, down-regulated Yap, suppressed proliferation, and promoted apoptosis in PAH, but not control PAVSMCs. PAVSM in small remodeled PAs from rats and mice with SU5416/hypoxia-induced PH showed down-regulation of LATS1 and overexpression of ILK1. Treatment of mice with selective ILK inhibitor Cpd22 at Days 22-35 of SU5416/hypoxia exposure restored LATS1 signaling and reduced established pulmonary vascular remodeling and PH.
Conclusions: These data report inactivation of HIPPO/LATS1, self-supported via Yap-fibronectin-ILK1 signaling loop, as a novel mechanism of self-sustaining proliferation and apoptosis resistance of PAVSMCs in PAH and suggest a new potential target for therapeutic intervention.
Keywords: HIPPO/LATS1; ILK; PAH; proliferation/apoptosis imbalance; vascular smooth muscle.
Figures


References
-
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(Suppl. S):13S–24S. - PubMed
-
- Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–874. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

