Optics-Integrated Microfluidic Platforms for Biomolecular Analyses
- PMID: 27119629
- PMCID: PMC4850344
- DOI: 10.1016/j.bpj.2016.03.018
Optics-Integrated Microfluidic Platforms for Biomolecular Analyses
Abstract
Compared with conventional optical methods, optics implemented on microfluidic chips provide small, and often much cheaper ways to interrogate biological systems from the level of single molecules up to small model organisms. The optical probing of single molecules has been used to investigate the mechanical properties of individual biological molecules; however, multiplexing of these measurements through microfluidics and nanofluidics confers many analytical advantages. Optics-integrated microfluidic systems can significantly simplify sample processing and allow a more user-friendly experience; alignments of on-chip optical components are predetermined during fabrication and many purely optical techniques are passively controlled. Furthermore, sample loss from complicated preparation and fluid transfer steps can be virtually eliminated, a particularly important attribute for biological molecules at very low concentrations. Excellent fluid handling and high surface area/volume ratios also contribute to faster detection times for low abundance molecules in small sample volumes. Although integration of optical systems with classical microfluidic analysis techniques has been limited, microfluidics offers a ready platform for interrogation of biophysical properties. By exploiting the ease with which fluids and particles can be precisely and dynamically controlled in microfluidic devices, optical sensors capable of unique imaging modes, single molecule manipulation, and detection of minute changes in concentration of an analyte are possible.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
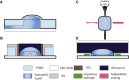
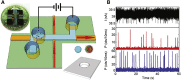
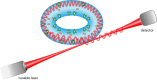

Similar articles
-
Micro-optics for microfluidic analytical applications.Chem Soc Rev. 2018 Feb 19;47(4):1391-1458. doi: 10.1039/c5cs00649j. Chem Soc Rev. 2018. PMID: 29308474 Review.
-
Microfluidic-integrated biosensors: prospects for point-of-care diagnostics.Biotechnol J. 2013 Nov;8(11):1267-79. doi: 10.1002/biot.201200386. Epub 2013 Sep 6. Biotechnol J. 2013. PMID: 24019250 Review.
-
Integrated microfluidic systems.Adv Biochem Eng Biotechnol. 2010;119:179-94. doi: 10.1007/10_2010_68. Adv Biochem Eng Biotechnol. 2010. PMID: 20535602 Review.
-
Microfluidic-integrated DNA nanobiosensors.Biosens Bioelectron. 2016 Nov 15;85:247-260. doi: 10.1016/j.bios.2016.05.009. Epub 2016 May 4. Biosens Bioelectron. 2016. PMID: 27179566 Review.
-
An integrated optics microfluidic device for detecting single DNA molecules.Lab Chip. 2007 Dec;7(12):1767-74. doi: 10.1039/b710504e. Epub 2007 Sep 27. Lab Chip. 2007. PMID: 18030399
Cited by
-
Roadmap on optical sensors.J Opt. 2024 Jan 1;26(1):013001. doi: 10.1088/2040-8986/ad0e85. Epub 2023 Dec 18. J Opt. 2024. PMID: 38116399 Free PMC article. Review.
-
Microfluidics-Based Nanobiosensors for Healthcare Monitoring.Mol Biotechnol. 2024 Mar;66(3):378-401. doi: 10.1007/s12033-023-00760-9. Epub 2023 May 11. Mol Biotechnol. 2024. PMID: 37166577 Free PMC article. Review.
-
Radiative decay engineering 8: Coupled emission microscopy for lens-free high-throughput fluorescence detection.Anal Biochem. 2017 Aug 15;531:20-36. doi: 10.1016/j.ab.2017.05.020. Epub 2017 May 17. Anal Biochem. 2017. PMID: 28527910 Free PMC article.
-
Analytic model for the complex effective index of the leaky modes of tube-type anti-resonant hollow core fibers.Sci Rep. 2017 Sep 18;7(1):11761. doi: 10.1038/s41598-017-12234-5. Sci Rep. 2017. PMID: 28924224 Free PMC article.
-
Label-Free Physical Techniques and Methodologies for Proteins Detection in Microfluidic Biosensor Structures.Biomedicines. 2022 Jan 18;10(2):207. doi: 10.3390/biomedicines10020207. Biomedicines. 2022. PMID: 35203416 Free PMC article. Review.
References
-
- Zhou J., Giridhar P.V., Papautsky I. Modulation of aspect ratio for complete separation in an inertial microfluidic channel. Lab Chip. 2013;13:1919–1929. - PubMed
-
- Di Carlo D. Inertial microfluidics. Lab Chip. 2009;9:3038–3046. - PubMed
-
- Loutherback K., Puchalla J., Sturm J.C. Deterministic microfluidic ratchet. Phys. Rev. Lett. 2009;102:045301-1–045301-4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

