Pyomelanin-producing Pseudomonas aeruginosa selected during chronic infections have a large chromosomal deletion which confers resistance to pyocins
- PMID: 27119970
- PMCID: PMC5295658
- DOI: 10.1111/1462-2920.13336
Pyomelanin-producing Pseudomonas aeruginosa selected during chronic infections have a large chromosomal deletion which confers resistance to pyocins
Abstract
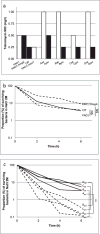
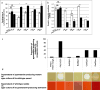
When bacterial lineages make the transition from free-living to permanent association with hosts, they can undergo massive gene losses, for which the selective forces within host tissues are unknown. We identified here melanogenic clinical isolates of Pseudomonas aeruginosa with large chromosomal deletions (66 to 270 kbp) and characterized them to investigate how they were selected. When compared with their wild-type parents, melanogenic mutants (i) exhibited a lower fitness in growth conditions found in human tissues, such as hyperosmolarity and presence of aminoglycoside antibiotics, (ii) narrowed their metabolic spectrum with a growth disadvantage with particular carbon sources, including aromatic amino acids and acyclic terpenes, suggesting a reduction of metabolic flexibility. Despite an impaired fitness in rich media, melanogenic mutants can inhibit their wild-type parents and compete with them in coculture. Surprisingly, melanogenic mutants became highly resistant to two intraspecific toxins, the S-pyocins AP41 and S1. Our results suggest that pyocins produced within a population of infecting P. aeruginosa may have selected for bacterial mutants that underwent massive gene losses and that were adapted to the life in diverse bacterial communities in the human host. Intraspecific interactions may therefore be an important factor driving the continuing evolution of pathogens during host infections.
© 2016 Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of interests The authors have declared that no competing interests exist.
Figures



References
-
- Beloin C, Valle J, Latour-Lambert P, Faure P, Kzreminski M, Balestrino D, et al. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol Microbiol. 2004;51:659–674. - PubMed
-
- Bentley SD, Parkhill J. Comparative genomic structure of prokaryotes. Annu Rev Genet. 2004;38:771–791. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

