Susceptibility tensor imaging (STI) of the brain
- PMID: 27120169
- PMCID: PMC5083244
- DOI: 10.1002/nbm.3540
Susceptibility tensor imaging (STI) of the brain
Abstract
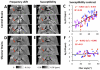
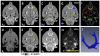
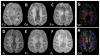
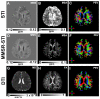
Susceptibility tensor imaging (STI) is a recently developed MRI technique that allows quantitative determination of orientation-independent magnetic susceptibility parameters from the dependence of gradient echo signal phase on the orientation of biological tissues with respect to the main magnetic field. By modeling the magnetic susceptibility of each voxel as a symmetric rank-2 tensor, individual magnetic susceptibility tensor elements as well as the mean magnetic susceptibility and magnetic susceptibility anisotropy can be determined for brain tissues that would still show orientation dependence after conventional scalar-based quantitative susceptibility mapping to remove such dependence. Similar to diffusion tensor imaging, STI allows mapping of brain white matter fiber orientations and reconstruction of 3D white matter pathways using the principal eigenvectors of the susceptibility tensor. In contrast to diffusion anisotropy, the main determinant factor of the susceptibility anisotropy in brain white matter is myelin. Another unique feature of the susceptibility anisotropy of white matter is its sensitivity to gadolinium-based contrast agents. Mechanistically, MRI-observed susceptibility anisotropy is mainly attributed to the highly ordered lipid molecules in the myelin sheath. STI provides a consistent interpretation of the dependence of phase and susceptibility on orientation at multiple scales. This article reviews the key experimental findings and physical theories that led to the development of STI, its practical implementations, and its applications for brain research. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: brain imaging; fiber tracking; gradient echo MRI; phase contrast; quantitative susceptibility mapping; susceptibility tensor imaging; white matter.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures


References
-
- Salomir R, De Senneville BD, Moonen CTW. A fast calculation method for magnetic field inhomogeneity due to an arbitrary distribution of bulk susceptibility. Concepts in Magnetic Resonance Part B-Magnetic Resonance Engineering. 2003;19B(1):26–34.
-
- Marques JP, Bowtell R. Application of a fourier-based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts in Magnetic Resonance Part B-Magnetic Resonance Engineering. 2005;25B(1):65–78.
-
- Liu T, Spincemaille P, de Rochefort L, Kressler B, Wang Y. Calculation of susceptibility through multiple orientation sampling (COSMOS): a method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magnetic Resonance in Medicine. 2009;61(1):196–204. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

