Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis
- PMID: 27120289
- PMCID: PMC5025835
- DOI: 10.1021/acs.chemrev.5b00760
Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis
Abstract
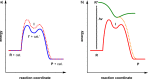
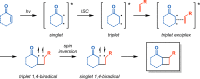
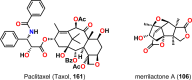
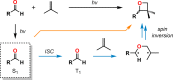
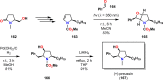
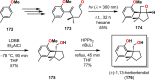
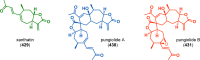
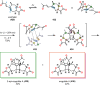
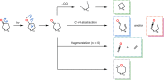
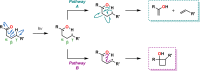
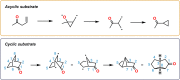
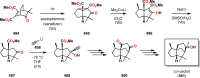
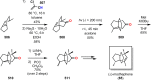
The use of photochemical transformations is a powerful strategy that allows for the formation of a high degree of molecular complexity from relatively simple building blocks in a single step. A central feature of all light-promoted transformations is the involvement of electronically excited states, generated upon absorption of photons. This produces transient reactive intermediates and significantly alters the reactivity of a chemical compound. The input of energy provided by light thus offers a means to produce strained and unique target compounds that cannot be assembled using thermal protocols. This review aims at highlighting photochemical transformations as a tool for rapidly accessing structurally and stereochemically diverse scaffolds. Synthetic designs based on photochemical transformations have the potential to afford complex polycyclic carbon skeletons with impressive efficiency, which are of high value in total synthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


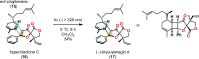

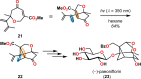
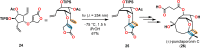
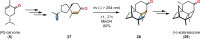
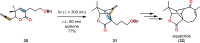


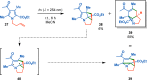
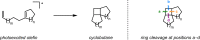
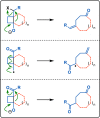
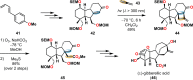
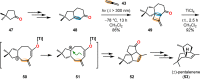
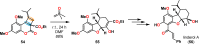
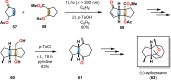
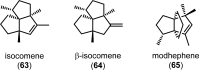
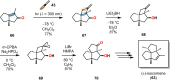

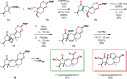
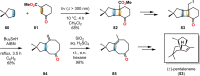
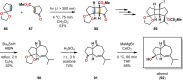
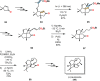
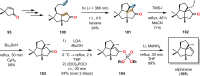
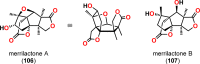


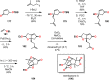
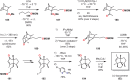
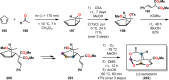
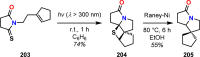
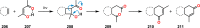

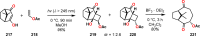
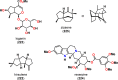
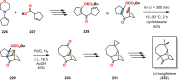
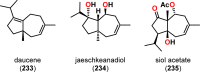
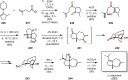
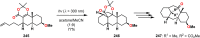
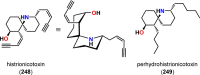
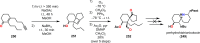
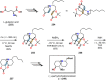
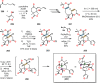
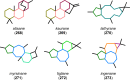
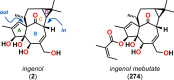
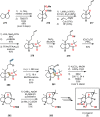
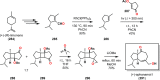

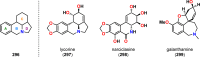


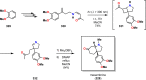
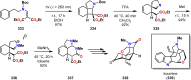
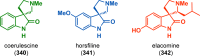
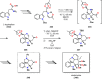

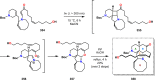
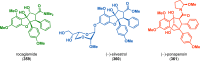
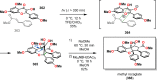
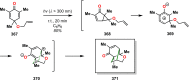
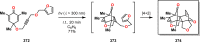
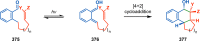
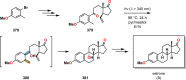
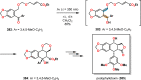
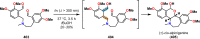
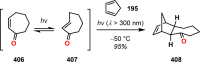


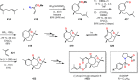
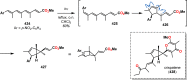
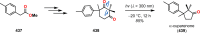
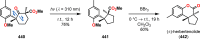
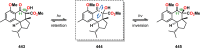

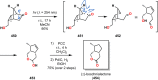
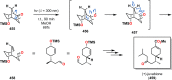
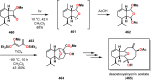
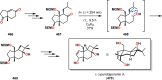
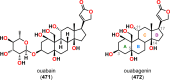
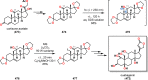
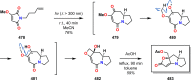
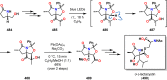
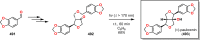
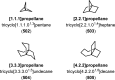
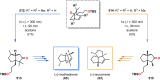
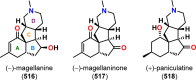
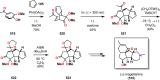
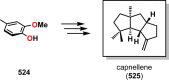


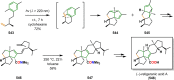
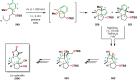
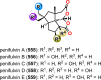
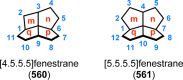
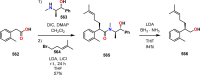
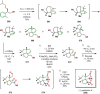
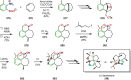


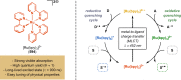
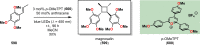

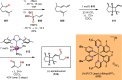
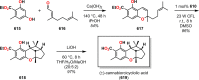
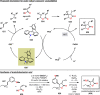
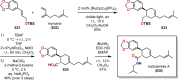
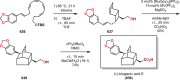
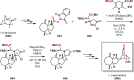
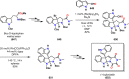
References
-
- Nicolaou K. C.; Chen J. S.. Classics in Total Synthesis III; Wiley-VCH: Weinheim, Germany, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

