Role of Rilpivirine and Etravirine in Efavirenz and Nevirapine-Based Regimens Failure in a Resource-Limited Country: A Cross- Sectional Study
- PMID: 27120449
- PMCID: PMC4847912
- DOI: 10.1371/journal.pone.0154221
Role of Rilpivirine and Etravirine in Efavirenz and Nevirapine-Based Regimens Failure in a Resource-Limited Country: A Cross- Sectional Study
Abstract
Introduction: Etravirine(ETR) can be used for patients who have failed NNRTI-based regimen. In Thailand, ETR is approximately 45 times more expensive than rilpivirine(RPV). However, there are no data of RPV use in NNRTI failure. Therefore, we assessed the susceptibility and mutation patterns of first line NNRTI failure and the possibility of using RPV compared to ETV in patients who have failed efavirenz(EFV)- and nevirapine(NVP)-based regimens.
Methods: Clinical samples with confirmed virological failure from EFV- or NVP-based regimens were retrospectively analyzed. Resistance-associated mutations (RAMs) were interpreted by IAS-USA Drug Resistance Mutations. Susceptibility of ETR and RPV were interpreted by DUET, Monogram scoring system, and Stanford University HIV Drug Resistance Database.
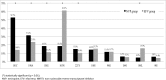
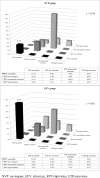
Results: 1,279 and 528 patients failed EFV- and NVP-based regimens, respectively. Y181C was the most common NVP-associated RAM (54.3% vs. 14.7%, p<0.01). K103N was the most common EFV-associated RAM (56.5% vs. 19.1%, P<0.01). The results from all three scoring systems were concordant. 165(11.1%) and 161(10.9%) patients who failed NVP-based regimen were susceptible to ETR and RPV, respectively (p = 0.85). 195 (32.2%) and 191 (31.6%) patients who failed EFV-based regimen, were susceptible to ETR and RPV, respectively (p = 0.79). The susceptibility of ETV and RPV in EFV failure was significantly higher than NVP failure (p<0.01).
Conclusion: The mutation patterns for ETR and RPV were similar but 32% and 11% of patients who failed EFV and NVP -based regimen, respectivly were susceptible to RPV. This finding suggests that RPV can be used as the alternative antiretroviral agent in patients who have failed EFV-based regimen.
Conflict of interest statement
Figures
References
-
- Adolescents PoAGfAa (2015) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services.
-
- (2013) Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach Geneva: World Health Organization - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical