HIV-free survival at 12-24 months in breastfed infants of HIV-infected women on antiretroviral treatment
- PMID: 27120500
- PMCID: PMC5096069
- DOI: 10.1111/tmi.12710
HIV-free survival at 12-24 months in breastfed infants of HIV-infected women on antiretroviral treatment
Abstract
Objective: To provide estimates of HIV-free survival at 12-24 months in breastfed children by maternal ART (6 months or lifelong) to inform WHO HIV and Infant Feeding guidelines.
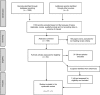
Methods: Eighteen studies published 2005-2015 were included in a systematic literature review (1295 papers identified, 156 abstracts screened, 55 full texts); papers were analysed by narrative synthesis and meta-analysis of HIV-free survival by maternal ART regimen in a random effects model. We also grouped studies by feeding modality. Study quality was assessed using a modified Newcastle-Ottawa Scale (NOS) and GRADE.
Results: The pooled estimates for 12-month HIV-free survival were 89.8% (95% confidence interval, CI: 86.5%, 93.2%) for infants of mothers on ART for 6 months post-natally (six studies) and 91.4% (95% CI 87.5%, 95.4%) for infants of mothers on lifelong ART (three studies). Eighteen-month HIV-free survival estimates were 89.0% (95% CI 83.9%, 94.2%) with 6 months ART (five studies) and 96.1% (95% CI 92.8%, 99.0%) with lifelong ART (three studies). Twenty-four-month HIV-free survival for infants whose mothers were on ART to 6 months post-natally (two studies) was 89.2% (95% CI 79.9%, 98.5%). Heterogeneity was considerable throughout. In four studies, HIV-free survival in breastfed infants ranged from 87% (95% CI 78%, 92%) to 96% (95% CI 91%, 98%) and in formula-fed infants from 67% (95% CI 35.5%, 87.9%) to 97.6% (95% CI 93.0%, 98.2%).
Conclusion: Our results highlight the importance of breastfeeding for infant survival and of ART in reducing the risk of mother-to-child HIV transmission and support the WHO recommendation to initiate ART for life immediately after HIV diagnosis.
Objectif:
Fournir des estimations sur la survie sans
Méthodes:
Dix‐huit études publiées entre 2005 et 2015 ont été incluses dans une analyse systématique de la littérature (1295 articles identifiés, 156 résumés screenés et 55 textes complets); les articles ont été analysés par synthèse narrative et une méta‐analyse de la survie sans
Résultats:
Les estimations poolées pour de la survie à 12 mois sans
Conclusion:
Nos résultats soulignent l'importance de l'allaitement maternel pour la survie du nourrisson et de l’
Objetivo:
Proveer cálculos sobre supervivencia a los 12‐24 meses, libre de
Métodos:
Se incluyeron dieciocho estudios publicados entre 2005‐2015, obtenidos mediante una revisión sistemática de la literatura (1295 artículos identificados, 156 resúmenes evaluados, 55 textos completos leídos); los artículos se analizaron mediante una síntesis narrativa y meta‐análisis de supervivencia libre de
Resultados:
Los cálculos de supervivencia sin
Conclusión:
Nuestros resultados subrayan la importancia de la lactancia para la supervivencia infantil y del
Keywords: ART; HIV-free survival; TAR; antiretroviral treatment; femmes; mujeres; revisión sistemática; revue systématique; supervivencia sin VIH; survie sans VIH; systematic review; women.
© 2016 The Authors. Tropical Medicine & International Health Published by John Wiley & Sons Ltd.
Figures




References
-
- WHO . Guidelines on HIV and Infant Feeding 2010: Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva, Switzerland: 2010. (Available from: http://apps.who.int/iris/bitstream/10665/44345/1/9789241599535_eng.pdf) [10 July 2015]. - PubMed
-
- WHO . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV Infenction: What's new. Geneva, Switzerland: 2015. (Available from: http://www.who.int/hiv/pub/guidelines/arv2013/en/) [10 July 2015].
-
- Wells GA, Shea B, O'Connell D et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta analysis 2000. (Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) [5 Sept 2015].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

