Glutamic Acid Residues in HIV-1 p6 Regulate Virus Budding and Membrane Association of Gag
- PMID: 27120610
- PMCID: PMC4848609
- DOI: 10.3390/v8040117
Glutamic Acid Residues in HIV-1 p6 Regulate Virus Budding and Membrane Association of Gag
Abstract
The HIV-1 Gag p6 protein regulates the final abscission step of nascent virions from the cell membrane by the action of its two late (L-) domains, which recruit Tsg101 and ALIX, components of the ESCRT system. Even though p6 consists of only 52 amino acids, it is encoded by one of the most polymorphic regions of the HIV-1 gag gene and undergoes various posttranslational modifications including sumoylation, ubiquitination, and phosphorylation. In addition, it mediates the incorporation of the HIV-1 accessory protein Vpr into budding virions. Despite its small size, p6 exhibits an unusually high charge density. In this study, we show that mutation of the conserved glutamic acids within p6 increases the membrane association of Pr55 Gag followed by enhanced polyubiquitination and MHC-I antigen presentation of Gag-derived epitopes, possibly due to prolonged exposure to membrane bound E3 ligases. The replication capacity of the total glutamic acid mutant E0A was almost completely impaired, which was accompanied by defective virus release that could not be rescued by ALIX overexpression. Altogether, our data indicate that the glutamic acids within p6 contribute to the late steps of viral replication and may contribute to the interaction of Gag with the plasma membrane.
Keywords: ">l-domains; ALIX; ESCRT; Gag p6; HIV-1; Tsg101; membrane association; ubiquitination; virus budding.
Figures

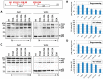
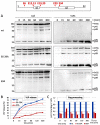
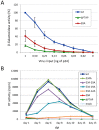

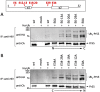




Similar articles
-
The HIV-1 gag p6: a promising target for therapeutic intervention.Retrovirology. 2024 Jan 23;21(1):1. doi: 10.1186/s12977-024-00633-2. Retrovirology. 2024. PMID: 38263239 Free PMC article. Review.
-
Ubiquitin conjugation to Gag is essential for ESCRT-mediated HIV-1 budding.Retrovirology. 2013 Jul 29;10:79. doi: 10.1186/1742-4690-10-79. Retrovirology. 2013. PMID: 23895345 Free PMC article.
-
The S40 residue in HIV-1 Gag p6 impacts local and distal budding determinants, revealing additional late domain activities.Retrovirology. 2013 Nov 21;10:143. doi: 10.1186/1742-4690-10-143. Retrovirology. 2013. PMID: 24257210 Free PMC article.
-
Basic residues in the nucleocapsid domain of Gag are critical for late events of HIV-1 budding.J Virol. 2011 Mar;85(5):2304-15. doi: 10.1128/JVI.01562-10. Epub 2010 Dec 15. J Virol. 2011. PMID: 21159863 Free PMC article.
-
Post-Translational Modifications of Retroviral HIV-1 Gag Precursors: An Overview of Their Biological Role.Int J Mol Sci. 2021 Mar 11;22(6):2871. doi: 10.3390/ijms22062871. Int J Mol Sci. 2021. PMID: 33799890 Free PMC article. Review.
Cited by
-
How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain.Viruses. 2020 Aug 13;12(8):888. doi: 10.3390/v12080888. Viruses. 2020. PMID: 32823718 Free PMC article. Review.
-
Exploring salivary metabolome alterations in people with HIV: towards early diagnostic markers.Front Public Health. 2024 Jun 5;12:1400332. doi: 10.3389/fpubh.2024.1400332. eCollection 2024. Front Public Health. 2024. PMID: 38912274 Free PMC article.
-
Proteolysis of mature HIV-1 p6 Gag protein by the insulin-degrading enzyme (IDE) regulates virus replication in an Env-dependent manner.PLoS One. 2017 Apr 7;12(4):e0174254. doi: 10.1371/journal.pone.0174254. eCollection 2017. PLoS One. 2017. PMID: 28388673 Free PMC article. Clinical Trial.
-
Calcium Contributes to Polarized Targeting of HIV Assembly Machinery by Regulating Complex Stability.JACS Au. 2022 Feb 1;2(2):522-530. doi: 10.1021/jacsau.1c00563. eCollection 2022 Feb 28. JACS Au. 2022. PMID: 35253001 Free PMC article.
-
The HIV-1 gag p6: a promising target for therapeutic intervention.Retrovirology. 2024 Jan 23;21(1):1. doi: 10.1186/s12977-024-00633-2. Retrovirology. 2024. PMID: 38263239 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

