Regulation of metabolic health and adipose tissue function by group 2 innate lymphoid cells
- PMID: 27120716
- PMCID: PMC5052033
- DOI: 10.1002/eji.201545562
Regulation of metabolic health and adipose tissue function by group 2 innate lymphoid cells
Abstract
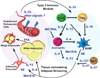
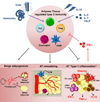
Adipose tissue (AT) is home to an abundance of immune cells. With chronic obesity, inflammatory immune cells accumulate and promote insulin resistance and the progression to type 2 diabetes mellitus. In contrast, recent studies have highlighted the regulation and function of immune cells in lean, healthy AT, including those associated with type 2 or "allergic" immunity. Although traditionally activated by infection with multicellular helminthes, AT type 2 immunity is active independently of infection, and promotes tissue homeostasis, AT "browning," and systemic insulin sensitivity, protecting against obesity-induced metabolic dysfunction and type 2 diabetes mellitus. In particular, group 2 innate lymphoid cells (ILC2s) are integral regulators of AT type 2 immunity, producing the cytokines interleukin-5 and IL-13, promoting eosinophils and alternatively activated macrophages, and cooperating with and promoting AT regulatory T (Treg) cells. In this review, we focus on the recent developments in our understanding of group 2 innate lymphoid cell cells and type 2 immunity in AT metabolism and homeostasis.
Keywords: Adipose tissue; Diabetes; Group 2 innate lymphoid cells; Metabolism; Type 2 immunity.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures
References
-
- Wensveen FM, Valentic S, Sestan M, Turk Wensveen T, Polic B. The "Big Bang" in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45:2446–2456. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

