Generation of siRNA Nanosheets for Efficient RNA Interference
- PMID: 27120975
- PMCID: PMC4848498
- DOI: 10.1038/srep25146
Generation of siRNA Nanosheets for Efficient RNA Interference
Abstract
After the discovery of small interference RNA (siRNA), nanostructured siRNA delivery systems have been introduced to achieve an efficient regulation of the target gene expression. Here we report a new siRNA-generating two dimensional nanostructure in a formation of nanosized sheet. Inspired by tunable mechanical and functional properties of the previously reported RNA membrane, siRNA nanosized sheets (siRNA-NS) with multiple Dicer cleavage sites were prepared. The siRNA-NS has two dimensional structure, providing a large surface area for Dicer to cleave the siRNA-NS for the generation of functional siRNAs. Furthermore, downregulation of the cellular target gene expression was achieved by delivery of siRNA-NS without chemical modification of RNA strands or conjugation to other substances.
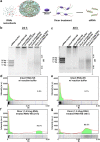
Figures



References
-
- Breaker R. R. Natural and engineered nucleic acids as tools to explore biology. Nature 432, 838–845 (2004). - PubMed
-
- Grabow W. W. & Jaeger L. RNA self-assembly and RNA nanotechnology. Acc Chem Res 47, 1871–1880 (2014). - PubMed
-
- Kim H. et al.. Nucleic acid engineering: RNA following the trail of DNA. ACS Comb Sci 18, 87–99 (2016). - PubMed
-
- Fire A. et al.. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

