Affect school and script analysis versus basic body awareness therapy in the treatment of psychological symptoms in patients with diabetes and high HbA1c concentrations: two study protocols for two randomized controlled trials
- PMID: 27121185
- PMCID: PMC4848779
- DOI: 10.1186/s13063-016-1347-8
Affect school and script analysis versus basic body awareness therapy in the treatment of psychological symptoms in patients with diabetes and high HbA1c concentrations: two study protocols for two randomized controlled trials
Abstract
Background: Depression is linked with alexithymia, anxiety, high HbA1c concentrations, disturbances of cortisol secretion, increased prevalence of diabetes complications and all-cause mortality. The psycho-educational method 'affect school with script analysis' and the mind-body therapy 'basic body awareness treatment' will be trialled in patients with diabetes, high HbA1c concentrations and psychological symptoms. The primary outcome measure is change in symptoms of depression. Secondary outcome measures are changes in HbA1c concentrations, midnight salivary cortisol concentration, symptoms of alexithymia, anxiety, self-image measures, use of antidepressants, incidence of diabetes complications and mortality.
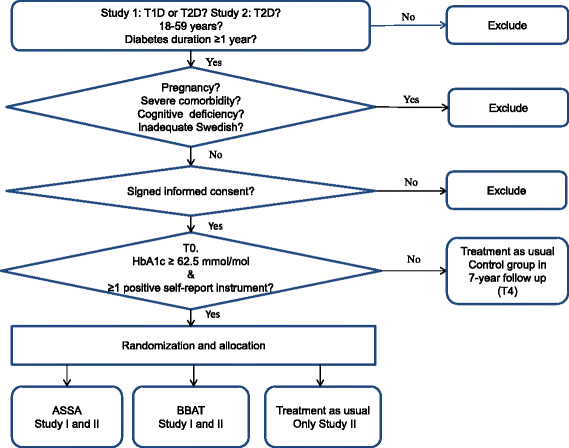
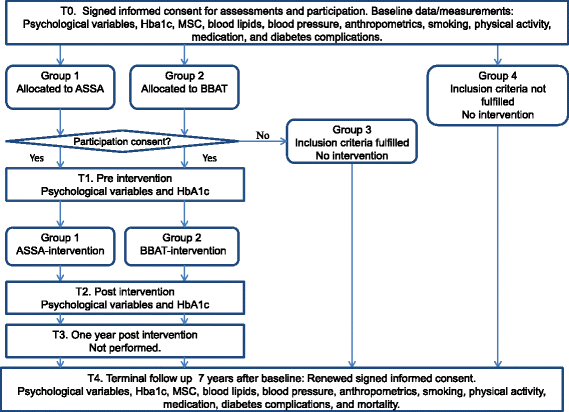
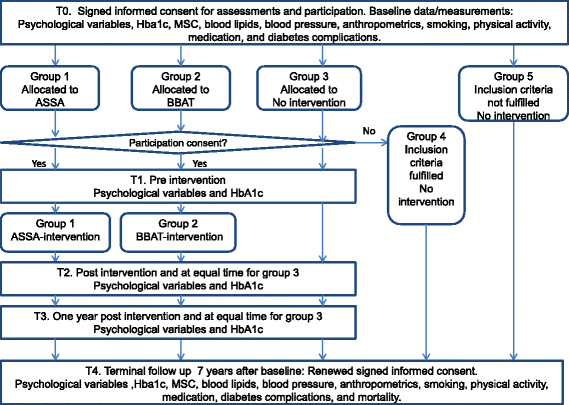
Methods: Two studies will be performed. Study I is an open-labeled parallel-group study with a two-arm randomized controlled trial design. Patients are randomized to either affect school with script analysis or to basic body awareness treatment. According to power calculations, 64 persons are required in each intervention arm at the last follow-up session. Patients with type 1 or type 2 diabetes were recruited from one hospital diabetes outpatient clinic in 2009. The trial will be completed in 2016. Study II is a multicentre open-labeled parallel-group three-arm randomized controlled trial. Patients will be randomized to affect school with script analysis, to basic body awareness treatment, or to treatment as usual. Power calculations show that 70 persons are required in each arm at the last follow-up session. Patients with type 2 diabetes will be recruited from primary care. This study will start in 2016 and finish in 2023. For both studies, the inclusion criteria are: HbA1c concentration ≥62.5 mmol/mol; depression, alexithymia, anxiety or a negative self-image; age 18-59 years; and diabetes duration ≥1 year. The exclusion criteria are pregnancy, severe comorbidities, cognitive deficiencies or inadequate Swedish. Depression, anxiety, alexithymia and self-image are assessed using self-report instruments. HbA1c concentration, midnight salivary cortisol concentration, blood pressure, serum lipid concentrations and anthropometrics are measured. Data are collected from computerized medical records and the Swedish national diabetes and causes of death registers.
Discussion: Whether the "affect school with script analysis" will reduce psychological symptoms, increase emotional awareness and improve diabetes related factors will be tried, and compared to "basic body awareness treatment" and treatment as usual.
Trial registration: ClinicalTrials.gov: NCT01714986.
Keywords: Alexithymia; Anxiety; Cortisol; Depression; Diabetes mellitus; HbA1c; Mind-body therapy; Psycho-education; Randomized controlled trial.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical