Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice
- PMID: 27121311
- PMCID: PMC5058752
- DOI: 10.18632/oncotarget.8886
Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice
Abstract
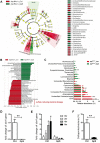
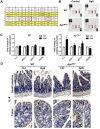
Saponins derived from medicinal plants have raised considerable interest for their preventive roles in various diseases. Here, we investigated the impacts of triterpenoid saponins isolated from Gynostemma pentaphyllum (GpS) on gut microbiome, mucosal environment, and the preventive effect on tumor growth. Six-week old ApcMin/+ mice and their wild-type littermates were fed either with vehicle or GpS daily for the duration of 8 weeks. The fecal microbiome was analyzed by enterobacterial repetitive intergenic consensus (ERIC)-PCR and 16S rRNA gene pyrosequencing. Study showed that GpS treatment significantly reduced the number of intestinal polyps in a preventive mode. More importantly, GpS feeding strikingly reduced the sulfate-reducing bacteria lineage, which are known to produce hydrogen sulfide and contribute to damage the intestinal epithelium or even promote cancer progression. Meanwhile, GpS also boosted the beneficial microbes. In the gut barrier of the ApcMin/+ mice, GpS treatment increased Paneth and goblet cells, up-regulated E-cadherin and down-regulated N-cadherin. In addition, GpS decreased the pro-oncogenic β-catenin, p-Src and the p-STAT3. Furthermore, GpS might also improve the inflamed gut epithelium of the ApcMin/+ mice by upregulating the anti-inflammatory cytokine IL-4, while downregulating pro-inflammatory cytokines TNF-α, IL-1β and IL-18. Intriguingly, GpS markedly stimulated M2 and suppressed M1 macrophage markers, indicating that GpS altered mucosal cytokine profile in favor of the M1 to M2 macrophages switching, facilitating intestinal tissue repair. In conclusion, GpS might reverse the host's inflammatory phenotype by increasing beneficial bacteria, decreasing sulfate-reducing bacteria, and alleviating intestinal inflammatory gut environment, which might contribute to its cancer preventive effects.
Keywords: gut microbiota; gut mucosal environment; gynostemma pentaphyllum; herbal saponins; pyrosequencing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Chen HM, Yu YN, Wang JL, Lin YW, Kong X, Yang CQ, Yang L, Liu ZJ, Yuan YZ, Liu F, Wu JX, Zhong L, Fang DC, Zou W, Fang JY. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr. 2013;97:1044–1052. - PubMed
-
- Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, Karre K, Pettersson S, Greicius G. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33:1231–1238. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

