TET1 inhibits gastric cancer growth and metastasis by PTEN demethylation and re-expression
- PMID: 27121319
- PMCID: PMC5058759
- DOI: 10.18632/oncotarget.8900
TET1 inhibits gastric cancer growth and metastasis by PTEN demethylation and re-expression
Abstract
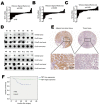
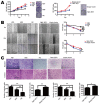
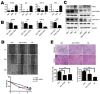
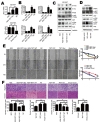
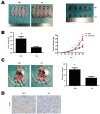
Ten-Eleven Translocation 1 (TET1) is a member of ten eleven translocation enzymes, which convert 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC). TET1 can promote CpG islands demethylation in specific genes and often absent in various cancers. Herein, we found that TET1 expression and 5-hmC content were low in gastric tumors compared to its adjacent non-tumor tissues. Cell proliferation, migration and invasion were enhanced upon TET1 knockdown in gastric cancer cells in vitro. This phenomenon was confirmed by an animal xeongraft model. We also found that TET1 directly binds to the promoter region of PTEN and activates its transcription through demethylation of CpG islands. TET1 knockdown activated AKT and FAK pathways, which were suppressed by PTEN. The activation of AKT and FAK facilitated tumor migration, invasion and accelerated cell growth. In conclusion, we found a novel mechanism that TET1 suppresses tumor cell growth, migration and invasion through demethylation of CpG island in PTEN promoter by increasing 5-hmC content. The re-expressed PTEN subsequently down regulates AKT and FAK activity.
Keywords: 5-hydroxymethylcytosine; 5-methylcytosine; PTEN; TET1; gastric cancer.
Conflict of interest statement
The authors declared no conflict of interest.
Figures


References
-
- Zhang H, Zhang X, Clark E, Mulcahey M, Huang S, Shi YG. TET1 is a DNA-binding protein that modulates DNA methylation and gene transcription via hydroxylation of 5-methylcytosine. Cell research. 2010;20:1390–1393. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

