Enantioselective CuH-Catalyzed Reductive Coupling of Aryl Alkenes and Activated Carboxylic Acids
- PMID: 27121395
- PMCID: PMC4866599
- DOI: 10.1021/jacs.6b03086
Enantioselective CuH-Catalyzed Reductive Coupling of Aryl Alkenes and Activated Carboxylic Acids
Abstract
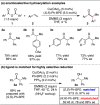
A new method for the enantioselective reductive coupling of aryl alkenes with activated carboxylic acid derivatives via copper hydride catalysis is described. Dual catalytic cycles are proposed, with a relatively fast enantioselective hydroacylation cycle followed by a slower diastereoselective ketone reduction cycle. Symmetrical aryl carboxyclic anhydrides provide access to enantioenriched α-substituted ketones or alcohols with excellent stereoselectivity and functional group tolerance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Corey E. J.; Kürti L.. Enantioselective Chemical Synthesis: Methods, Logic and Practice; Direct Book Publishing: Dallas, 2010.
-
-
For selected reviews on hydroacylation:
- Yang L.; Huang H. Chem. Rev. 2015, 115, 3468.10.1021/cr500610p. - DOI - PubMed
- Leung J. C.; Krische M. J. Chem. Sci. 2012, 3, 2202.10.1039/c2sc20350b. - DOI
- Willis M. C. Chem. Rev. 2010, 110, 725.10.1021/cr900096x. - DOI - PubMed
- Park Y. J.; Park J.-W.; Jun C.-H. Acc. Chem. Res. 2008, 41, 222.10.1021/ar700133y. - DOI - PubMed
-
-
-
For selected reviews on alkene reductive coupling:
- Ketcham J. M.; Shin I.; Montgomery T. P.; Krische M. J. Angew. Chem., Int. Ed. 2014, 53, 9142.10.1002/anie.201403873. - DOI - PMC - PubMed
- Dechert-Schmitt A.-M. R.; Schmitt D. C.; Gao X.; Itoh T.; Krische M. J. Nat. Prod. Rep. 2014, 31, 504.10.1039/c3np70076c. - DOI - PMC - PubMed
- Bower J. F.; Kim I. S.; Patman R. L.; Krische M. J. Angew. Chem., Int. Ed. 2009, 48, 34.10.1002/anie.200802938. - DOI - PMC - PubMed
- Ngai M.-Y.; Kong J.-R.; Krische M. J. J. Org. Chem. 2007, 72, 1063.10.1021/jo061895m. - DOI - PubMed
- Montgomery J.Organonickel Chemistry. In Organometallics in Synthesis; Lipshutz B. H., Ed.; John Wiley & Sons, Inc.: Hoboken, 2013; pp 319–428.
- Ng S.-S.; Ho C.-Y.; Schleicher K. D.; Jamison T. F. Pure Appl. Chem. 2008, 80, 929.10.1351/pac200880050929. - DOI - PMC - PubMed
- Reichard H. A.; Micalizio G. C. Chem. Sci. 2011, 2, 573.10.1039/C0SC00394H. - DOI - PMC - PubMed
- Micalizio G. C.; Hale S. B. Acc. Chem. Res. 2015, 48, 663.10.1021/ar500408e. - DOI - PMC - PubMed
- Ho C.-Y.; Schleicher K. D.; Chan C.-W.; Jamison T. F. Synlett 2009, 2565.10.1055/s-0029-1217747. - DOI - PMC - PubMed
- Sato F.; Urabe H.; Okamoto S. Chem. Rev. 2000, 100, 2835.10.1021/cr990277l. - DOI - PubMed
-
-
-
For reviews on asymmetric hydroacylation, see:
- Murphy S. K.; Dong V. M. Chem. Commun. 2014, 50, 13645.10.1039/C4CC02276A. - DOI - PMC - PubMed
- González-Rodríguez C.; Willis M. C. Pure Appl. Chem. 2011, 83, 577.10.1351/PAC-CON-10-09-23. - DOI
-
For selected enantioselective intermolecular hydroacylation reactions, see:
- Liu F.; Bugaut X.; Schedler M.; Fröhlich R.; Glorius F. Angew. Chem., Int. Ed. 2011, 50, 12626.10.1002/anie.201106155. - DOI - PubMed
- Stemmler R. T.; Bolm C. Adv. Synth. Catal. 2007, 349, 1185.10.1002/adsc.200600583. - DOI
- Shibata Y.; Tanaka K. J. Am. Chem. Soc. 2009, 131, 12552.10.1021/ja905908z. - DOI - PubMed
- Coulter M. M.; Kou K. G. M.; Galligan B.; Dong V. M. J. Am. Chem. Soc. 2010, 132, 16330.10.1021/ja107198e. - DOI - PubMed
- Phan D. H. T.; Kou K. G. M.; Dong V. M. J. Am. Chem. Soc. 2010, 132, 16354.10.1021/ja107738a. - DOI - PubMed
- Osborne J. D.; Randell-Sly H. E.; Currie G. S.; Cowley A. R.; Willis M. C. J. Am. Chem. Soc. 2008, 130, 17232.10.1021/ja8069133. - DOI - PubMed
-
-
-
For reviews on reductive aldol, Mannich, and Michael reactions:
- Nishiyama H.; Shiomi T. Top. Curr. Chem. 2007, 279, 105.10.1007/128_2007_126. - DOI
- Guo H.-C.; Ma J.-A. Angew. Chem., Int. Ed. 2006, 45, 354.10.1002/anie.200500195. - DOI - PubMed
- Jang H.-Y.; Krische M. J. Eur. J. Org. Chem. 2004, 3953.10.1002/ejoc.200400270. - DOI - PubMed
-
Selected Cu-catalyzed reductive coupling reactions:
- Lipshutz B. H.; Amorelli B.; Unger J. B. J. Am. Chem. Soc. 2008, 130, 14378.10.1021/ja8045475. - DOI - PubMed
- Zhao D.; Oisaki K.; Kanai M.; Shibasaki M. J. Am. Chem. Soc. 2006, 128, 14440.10.1021/ja0652565. - DOI - PubMed
- Deschamp J.; Chuzel O.; Hannedouche J.; Riant O. Angew. Chem., Int. Ed. 2006, 45, 1292.10.1002/anie.200503791. - DOI - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

