Dynamics of the fecal microbiome in patients with recurrent and nonrecurrent Clostridium difficile infection
- PMID: 27121861
- PMCID: PMC4847246
- DOI: 10.1186/s13073-016-0298-8
Dynamics of the fecal microbiome in patients with recurrent and nonrecurrent Clostridium difficile infection
Abstract
Background: Recurrent Clostridium difficile infection (CDI) remains problematic, with up to 30 % of individuals diagnosed with primary CDI experiencing at least one episode of recurrence. The success of microbial-based therapeutics, such as fecal microbiota transplantation, for the treatment of recurrent CDI underscores the importance of restoring the microbiota. However, few studies have looked at the microbial factors that contribute to the development of recurrent disease. Here we compare microbial changes over time in patients with or without recurrence to identify microbial signatures associated with the development of recurrence.
Methods: We used 16S rRNA-encoding gene sequence analysis to compare the fecal microbiota of 93 patients with recurrent and nonrecurrent CDI, sampled longitudinally. Cross-group and intra-individual differences in microbial community diversity and similarity were compared prior to the development of recurrent disease and over time.
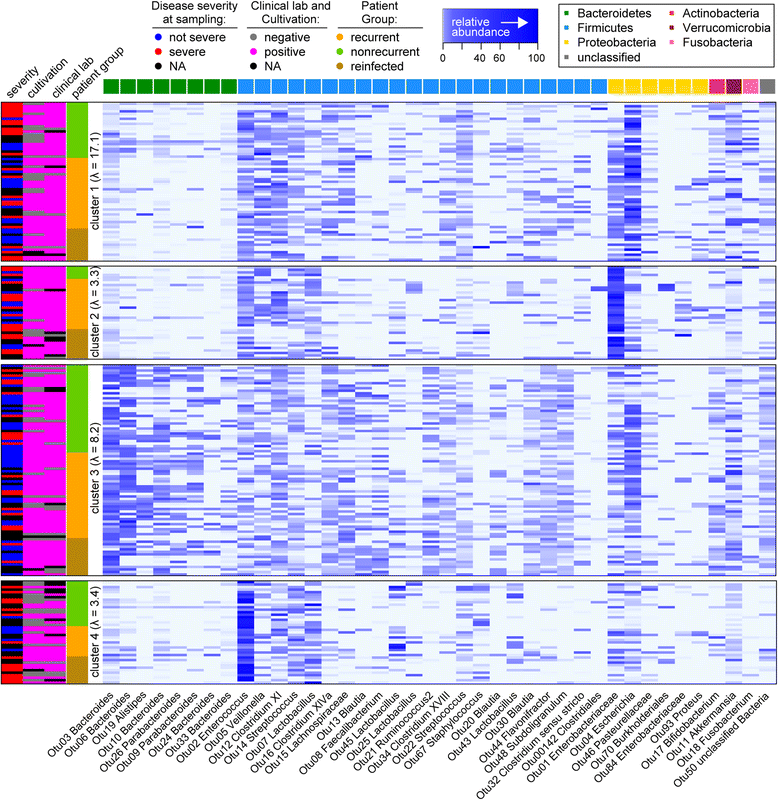
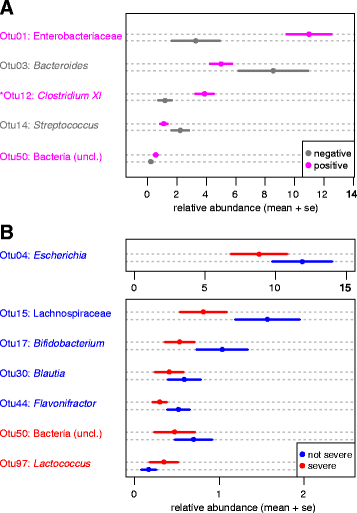
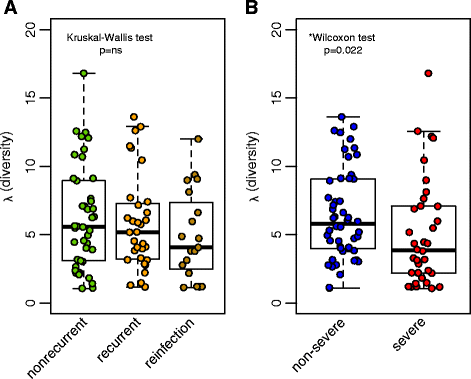
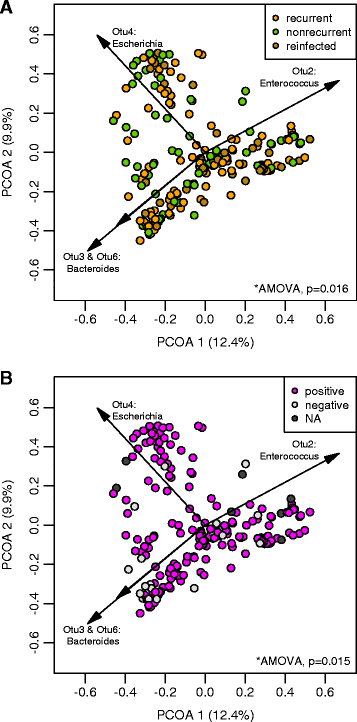
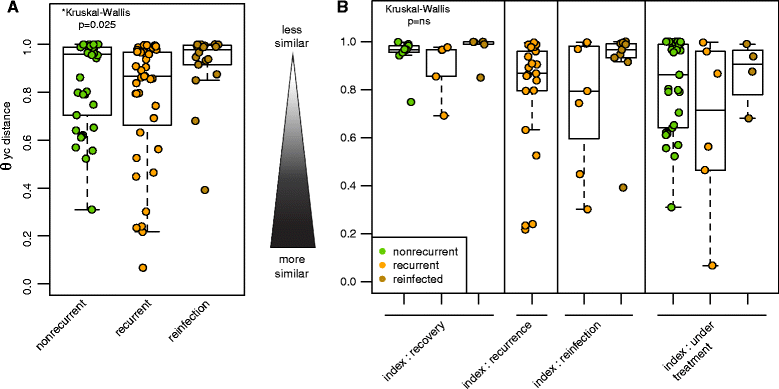
Results: Samples from these patient groups exhibited variable community profiles, clustering into four distinct community groups. Cross-group comparison of the index sample collected from patients that did or did not develop recurrence revealed differences in diversity and community structure (analysis of molecular variance, p < 0.05). Intra-individual comparisons of the microbiota were more informative and samples from recurrent patients were less likely to recover in diversity (Chi-square test, p < 0.005), exhibiting less community similarity overall (Kruskal-Wallis test, p < 0.05). Interestingly, patients with severe disease harbored a significantly less diverse community, a trend that was observed across both nonrecurrent and recurrent patient groups (Wilcoxon test, p < 0.05).
Conclusions: To date, this study represents one of the largest studies focused on the relationship between predictive signals from the gut microbiota and the development of recurrent CDI. Our data demonstrate that specific microbiota-derived characteristics associate with disease severity and recurrence and that future studies could incorporate these characteristics into predictive models.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

